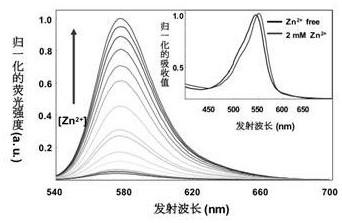
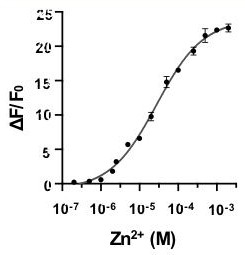
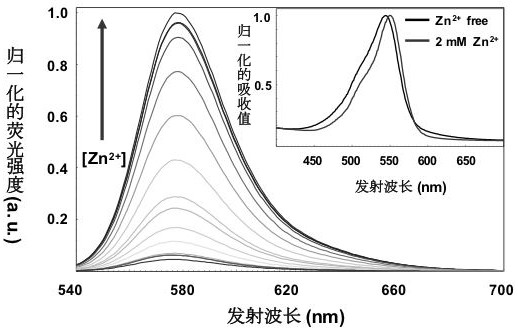
Rhodamine fluorescent dye containing water-soluble substituent as well as preparation method and application of rhodamine fluorescent dye
A fluorescent dye, water-soluble technology, used in its application in biological systems, can solve problems such as poor water solubility, high phototoxicity, and non-specific labeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060] Example 1: the synthesis of compound PK Zinc Red-1:
[0061] (1) Synthesis of Compound 1b:
[0062]
[0063] First, compound 1a (1.00 g, 3.39 mmol, 1.0 e.q.) was dissolved in 10 mL of dry DMF and stirred at -20°C for 30 min. Then, the POCl 3 (2.88 g, 18.8 mmol, 5.5 e.q.) was added dropwise to the above solution, and the temperature was raised to 60°C for 3 h. After the above reaction, the resulting solution was slowly dropped into 20 mL of ice-water mixture, followed by Na 2 CO 3 The solution neutralized the reaction solution and extracted three times with dichloromethane (40 mL each time), collected the organic layer, and washed with Na 2 SO 4 Solid dry. The resulting solution was concentrated under vacuum / reduced pressure, and purified by silica gel flash column chromatography (developing solvent: 10% ethyl acetate / petroleum ether) to obtain compound 1b (920 mg, 2.84 mmol, yield 84%) as a yellow solid.
[0064] 1 H NMR (400 MHz, Chloroform- d ) δ 9.78 (s, ...
example 2
[0079] Example 2: Synthesis of Compound PK Zinc Red-2:
[0080] (1) Synthesis of compound 2b:
[0081]
[0082] Firstly, compound 2a (730 mg, 2.36 mmol, 1.0 e.q.) was dissolved in 10 mL of dry DMF and stirred at -20°C for 30 min. Then, the POCl 3 (3.00 g, 19.6 mmol, 8.3 e.q.) was added dropwise to the above solution, and the resulting mixture was heated to 60°C for 3 h. After the above reaction was completed, the resulting solution was slowly dropped into 20 mL of ice-water mixture, and then the mixture was washed with Na 2 CO 3 The solution was neutralized and extracted three times with dichloromethane (40 mL each), and the organic layer was collected and washed with Na 2 SO 4 Solid dry. The resulting solution was concentrated under vacuum / reduced pressure, and purified by silica gel flash column chromatography (developing solvent 10% ethyl acetate / petroleum ether) to obtain compound 2b (680 mg, 2.02 mmol, yield 86%) as a yellow solid.
[0083] 1 H NMR (400 MHz, Ch...
example 3
[0098] Example 3: Synthesis of Compound PK Zinc Red-3:
[0099] (1) Synthesis of compound 3b:
[0100]
[0101] Firstly, compound 3a (700 mg, 2.06 mmol, 1.0 e.q.) was dissolved in 10 mL of dry DMF and stirred at -20°C for 30 min. Then, the POCl 3 (2.52 g, 16.5 mmol, 8.0 e.q.) was added dropwise to the above solution, and the resulting mixture was heated to 60°C for 3 h. After the above reaction was completed, the resulting solution was slowly dropped into 20 mL of ice-water mixture, and then the mixture was washed with Na 2 CO 3 The solution was neutralized and extracted three times with dichloromethane (40 mL each), and the organic layer was collected and washed with Na 2 SO 4 Solid dry. The resulting solution was concentrated under vacuum / reduced pressure, and purified by silica gel flash column chromatography (developing solvent 10% EtOAc / hexane) to obtain compound 3b (500 mg, 1.36 mmol, yield 66%) as a yellow oil.
[0102] 1 H NMR (400 MHz, Chloroform- d ) δ 9....
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com