Hydrophobic polysaccharide and its preparation method and application
A hydrophobization and polysaccharide technology, applied in the field of biomedicine, can solve the problems of high toxicity and side effects and limit clinical application, and achieve the effect of high pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
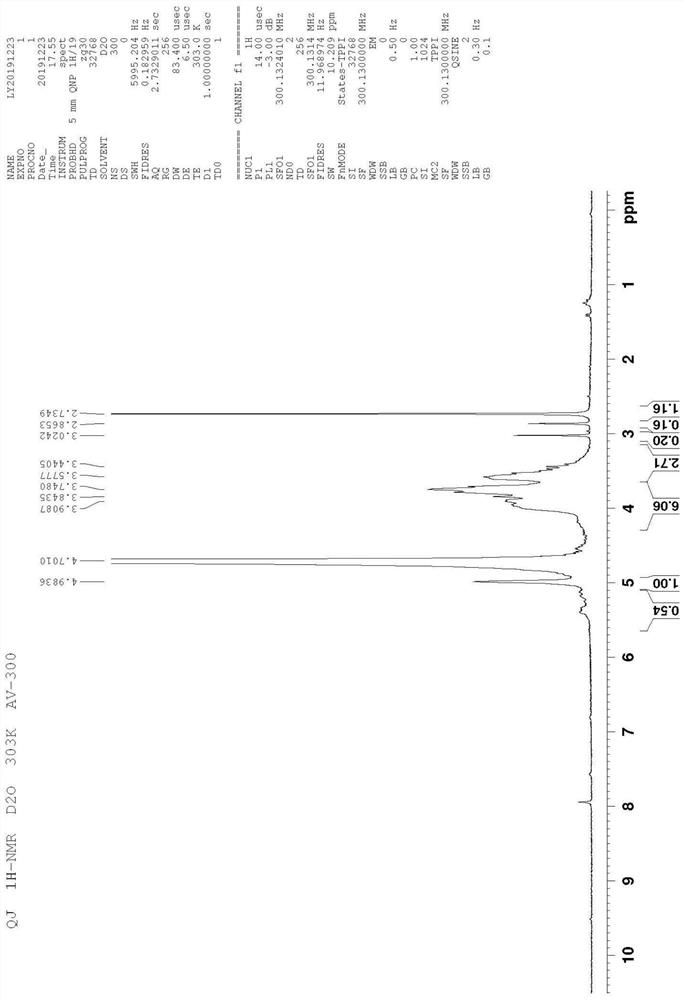
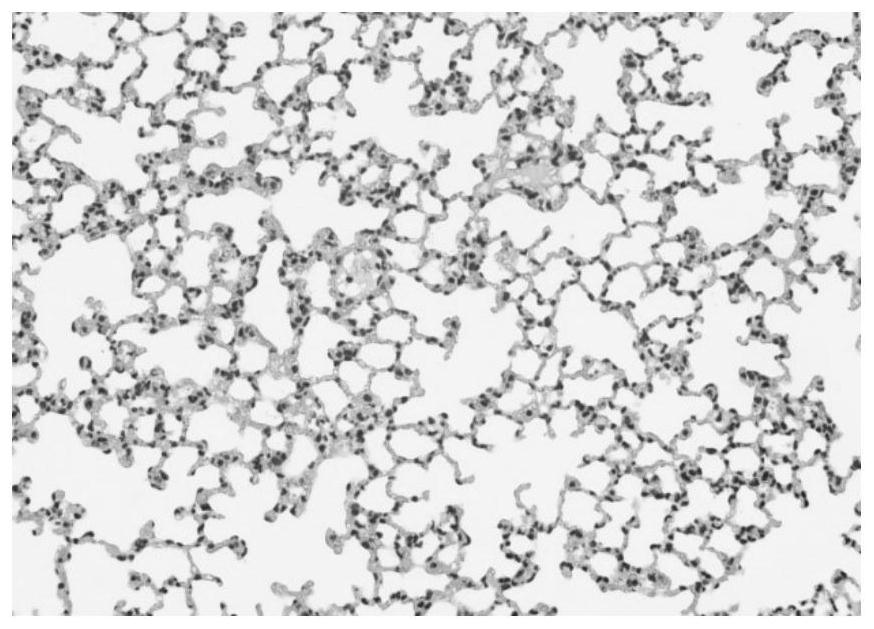
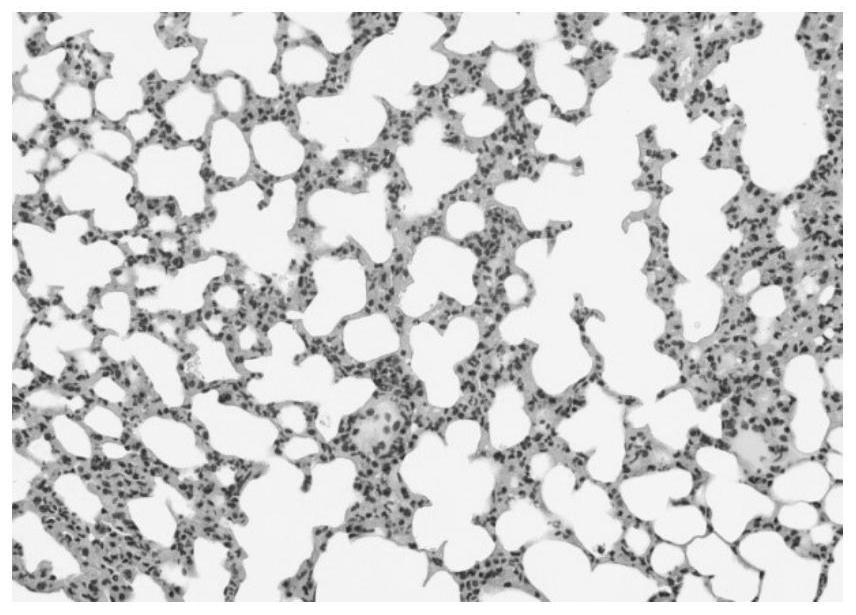
[0065] The present invention provides a novel hydrophobized polysaccharide, a preparation method thereof, and an application in pharmaceutical preparations as an immunomodulatory substance. Such hydrophobized polysaccharides (also known as hydrophobized polysaccharide modifications) have various physiological activities, such as anti-immunity and immune-enhancing activities. It can be used as a carrier of anti-inflammatory, antibacterial, antiviral, antitumor drugs, amino acids, peptides, and various anti-inflammatory drugs, various antibacterial drugs, various antiviral drugs, various antitumor drugs, various amino acids, various It is used together with various peptides for the treatment of various immune diseases. For example, various types of inflammation, various types of fungal infections, various types of viroid infections, various types of tumor treatment, treatment of diabetes, treatment of multiple sclerosis, including the treatment of immune diseases such as myasthe...
Embodiment 1
[0104] Example 1 Hydrophobized glucan modification and preparation method thereof
[0105] For the hydrophobized polysaccharide prepared in this example, the linking arm of diacetoxysuccinamide was first synthesized, and then the hydroxyl group of cholesterol reacted with the carboxyl group at one end of the linking arm to prepare cholesterol-esterified diacetoxysuccinamide, which was then reacted with dextran to obtain Hydrophobized dextran modifications. The specific preparation method is as follows:
[0106] 1. Dissolve 100g (0.01mol) succinic acid in 2L toluene, add 0.02mol carboxyl-protected glycine, add catalyst DMAP 1g and dehydrating agent DCC 10g, react at room temperature for 48h, filter to remove by-product dicyclohexylurea, add water to the filtrate A small amount of the reaction was terminated, and 2 L of absolute ethanol was added to obtain a white precipitate, which was diacetoxysuccinamide.
[0107] 2. Remove the protection of the carboxyl group of diacetoxys...
Embodiment 2
[0110] Example 2 Hydrophobized glucan modification and preparation method thereof
[0111] The specific preparation method is as follows:
[0112] 1. Dissolve 100g (0.02mol) succinic acid in 4L toluene, add 0.04mol carboxyl-protected glycine, add catalyst DMAP 2g and dehydrating agent DCC 20g, react at 40°C for 24h, filter to remove by-product dicyclohexylurea, the filtrate A small amount of water was added to terminate the reaction, and 4 L of absolute ethanol was added to obtain a white precipitate, which was diacetoxysuccinamide.
[0113] 2. Remove the protection of the carboxyl group of diacetoxysuccinamide. The specific method is as follows: add 100 g of diacetoxysuccinamide to 1 L of 1N NaOH aqueous solution for 2 hours at room temperature, and then add 50 mL of 1N HCl aqueous solution to neutralize the remaining hydrogen Sodium oxide to free the carboxyl group of diacetoxysuccinamide. After the reaction system was distilled under reduced pressure to remove water, it wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com