Application of Alfuzosin in preparation of medicine for treating diabetes and complications thereof
A technology of alfuzosin and diabetes, which is applied in the field of new application of alfuzosin in the preparation of drugs for the treatment of diabetes and its complications, can solve the problems of low cure rate of diabetes complications, achieve remarkable therapeutic effect, improve kidney Function, the effect of inhibiting cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Establishing 1 C57BL / 6N mouse diabetic model, and the packet Example
[0047] 1. The establishment of model
[0048] The 70 C57BL / 6N mice were randomly divided into three groups, i.e., normal food group (n = 20), high-fat diet group (n = 20), high-fat diet + STZ group (30).
[0049] Normal diet mice: normal diet after three weeks, 5 days of continuous injection of an equal volume of phosphate buffer solution, and then continue with a normal diet until the end of the modeling;
[0050] High fat diet mice: three weeks after high fat diet, continuous injection of 5 days an equal volume of phosphate buffer solution, and then continue with the high fat diet until the end of the modeling;
[0051] STZ + high-fat diet mice: three weeks after high fat diet, was dosed intraperitoneal injection of STZ 45mg / kg of water fasted 12h (before STZ injection, injection in accordance with the same time period 9:00 am-10:00am after fasted for 2h after injection of STZ finished can be fed...
Embodiment 2
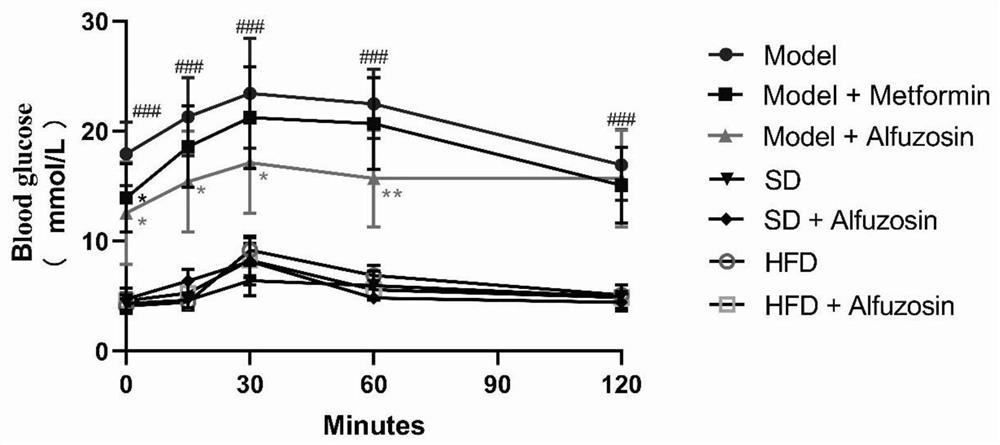
[0062] Example 2 index detection
[0063] The experimental data was statistically analyzed by SPSS23.0 software, and the data was expressed (x ± s), and the inter-group comparison was two-Way ANOVAs and LSD-T method for two comparisons. P <0.05 is considered to have statistical significance.
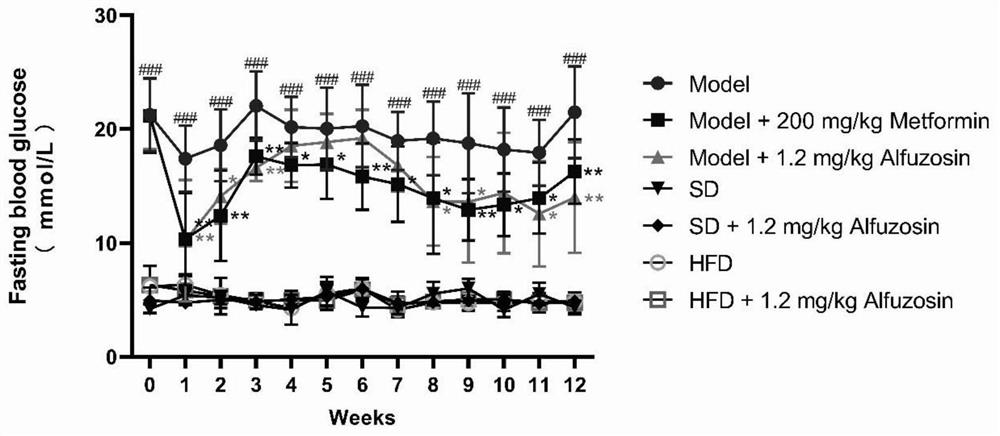
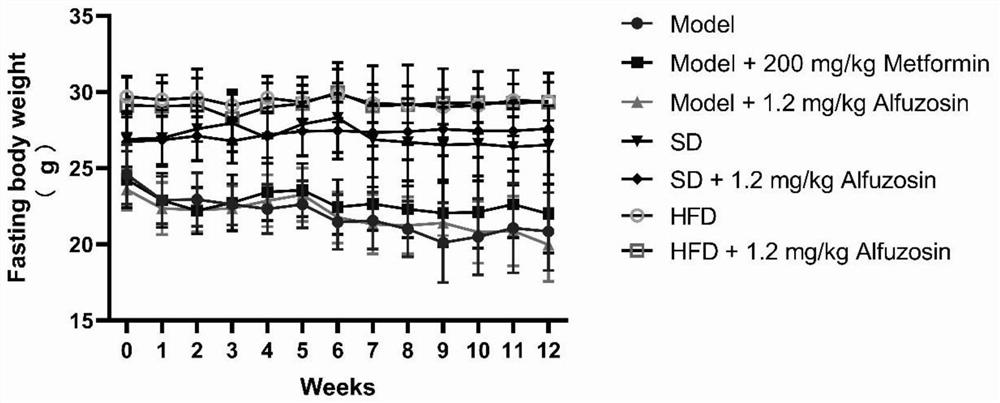
[0064] 1. body weight and fasting plasma glucose (Fasting Blood Glucose, FBG) monitoring
[0065] Dosing week 0, week fasted After 12h, water the same time period (7:30 pm-9:00pm) fasting blood glucose, blood amount tails, blood glucose content by the glucose meter, the blood glucose level reading recorded ; week before blood glucose measurement electronic weighing scales.
[0066] Effects of alfuzosin results such as fasting blood glucose in diabetic mice figure 1 Shown, wherein Model vs.SD, ### p ** p <0.01, 2 weeks 5 weeks blood glucose increased slightly, from the first six weeks to 12 weeks, Model + Alfuzosin blood glucose groups continued to decline, and lower than the positive control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com