Method for preparing 2,4-dichloroacetophenone
A technology of dichloroacetophenone and m-dichlorobenzene, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, which can solve the problems of low preparation yield and poor purity, and achieve simple synthesis process , Promote dissolution and reduce reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] In order to enable those skilled in the art to better understand the technical solutions in the present application, the technical solutions in the embodiments of the present application will be clearly and completely described below in conjunction with the accompanying drawings.
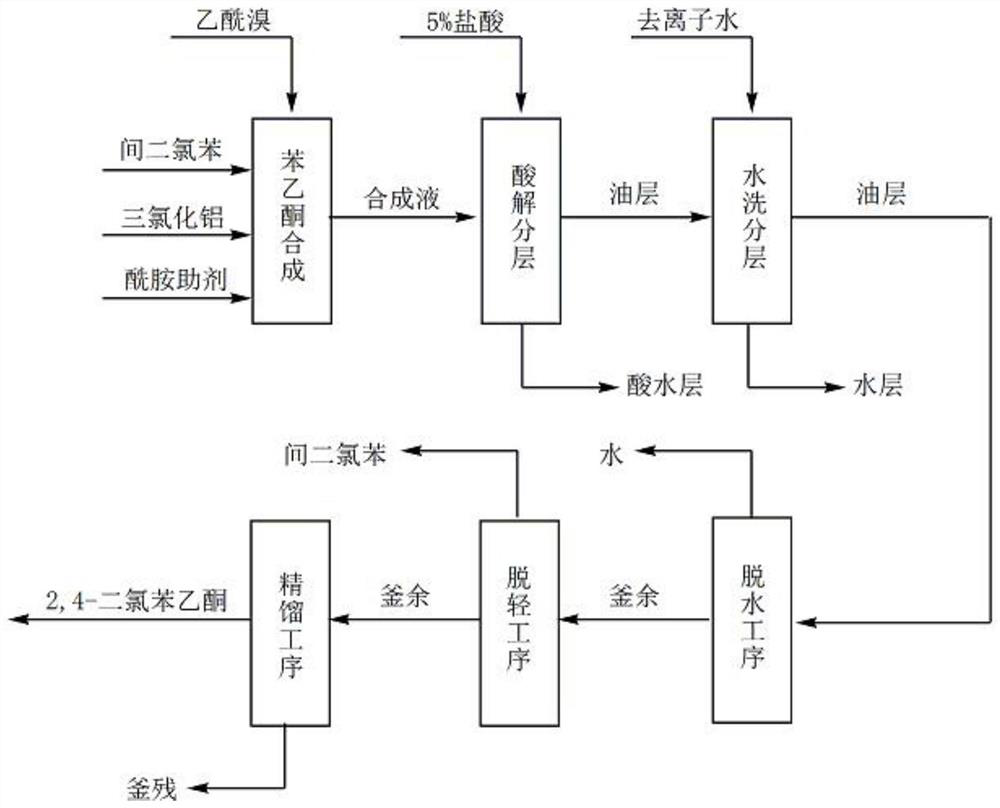
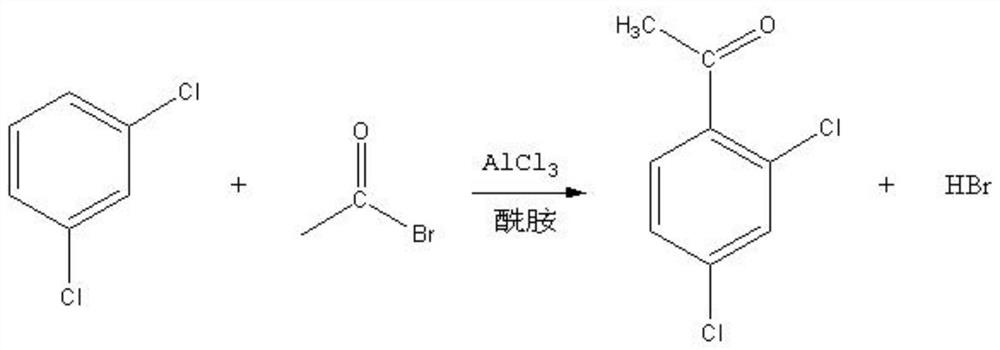
[0023] see Figure 1-2
[0024] Known by above technical scheme, 1, a kind of method for preparing 2,4-dichloroacetophenone comprises the following steps:
[0025] S1: Mix and stir dichlorobenzene, aluminum trichloride, and amide additives;
[0026] S2: When the stirring temperature rises to 40°C, add acetyl bromide dropwise to it for reaction, the dropwise addition process lasts for 2-3 hours and keep warm for 1-2 hours after the temperature is raised to 100°C, and when the temperature is lowered to 50°C, 2,4-dichloroacetophenone synthetic liquid;
[0027] S3: Add the 2,4-dichloroacetophenone synthesis liquid in step 2 to hydrochloric acid for acidolysis and layering, and separate the oil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com