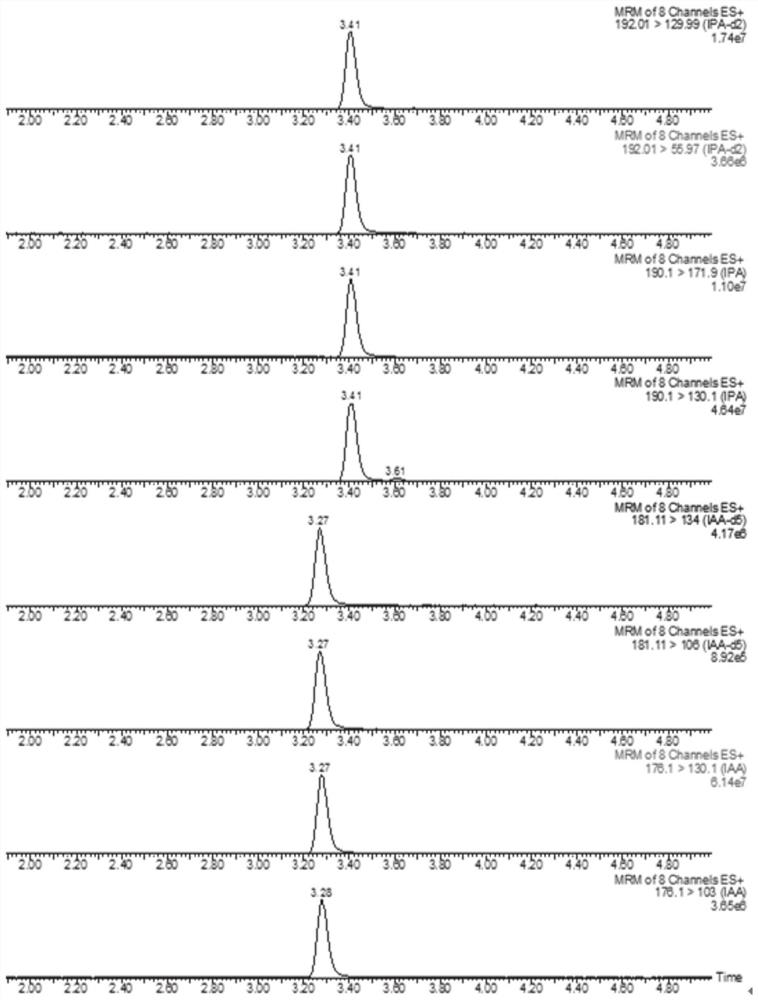
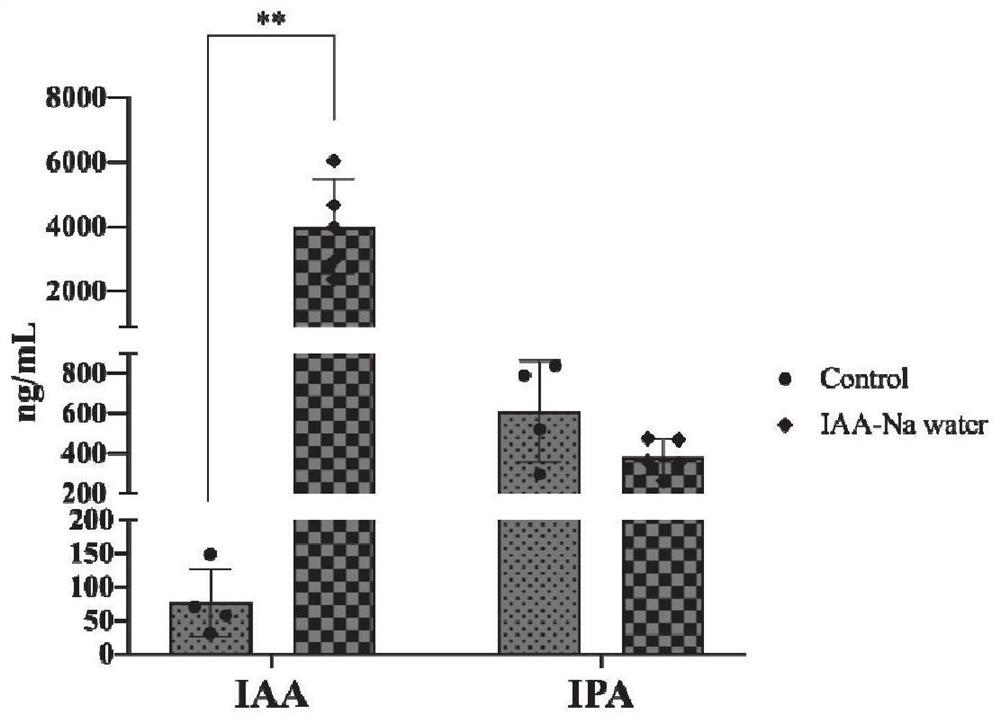
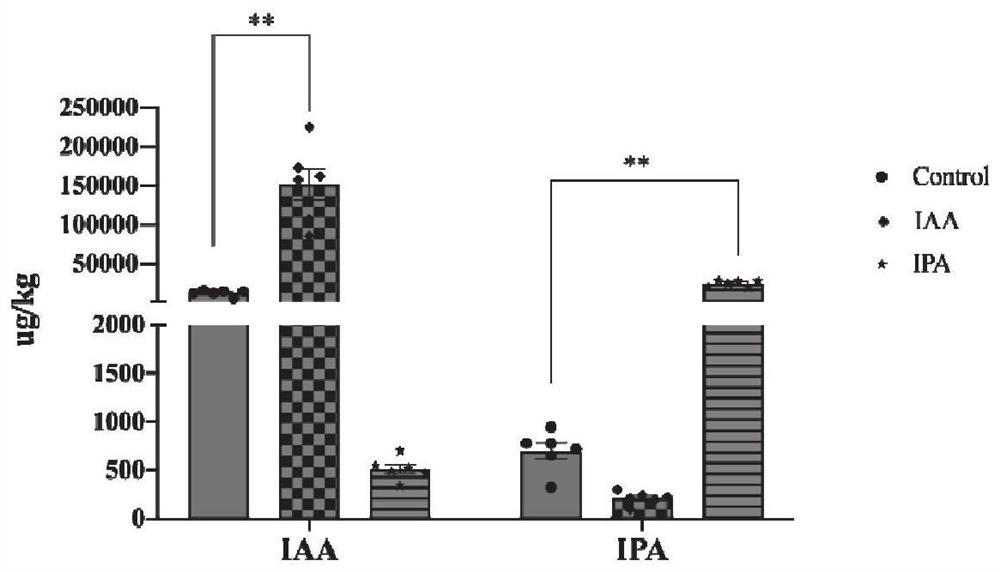
Method for detecting indoleacetic acid and indolepropionic acid in serum and excrement
A detection method and technology of indole acetic acid, applied in the field of metabolite detection, can solve the problems of high price, complex matrix, and certain difficulty in removal, and achieve the effect of simple pretreatment process and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Determination of indole acetic acid and indole propionic acid in mouse serum
[0046] 1. Ultrasonic solvent extraction: Use a pipette gun to accurately pipette 100 μL of mouse serum into a 2 mL EP tube, add an appropriate volume of internal standards (indole acetic acid-d5 and indole propionic acid-d2), add 0.45 mL of extraction solution (methanol : water: formic acid = 15: 4: 1), sealed and ultrasonically extracted for 15 min, centrifuged at 10,000 rpm and 4°C for 10 min, and after taking the supernatant, add 0.45 mL of extract (methanol: water: formic acid = 15: 4 : 1) Repeat the extraction once, and combine the extracts.
[0047] 2. Enrichment and purification of solid-phase extraction cartridges: first activate the Oasis HLB (3cc / 60mg) solid-phase extraction cartridges with 4mL methanol and 4mL water, then accurately pipette 600μL extract solution for loading, add 4mL water for rinsing , and then eluted with 3 mL of eluent (0.1% formic acid in methanol), and collec...
Embodiment 2
[0054] Determination of indole acetic acid and indole propionic acid in mouse feces
[0055] 1. Homogeneous grinding + ultrasonic solvent extraction: Accurately weigh 0.05g mouse feces sample into a 2mL EP tube, add an appropriate volume of internal standards (indole acetic acid-d5 and indole propionic acid-d2), add 0.5mL extract (methanol: water: formic acid = 15:4:1), add grinding beads, grind at 4°C, ultrasonically extract for 15 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C, take the supernatant, and then add 0.5mL The extract (methanol: water: formic acid = 15:4:1) was repeatedly extracted once, and the extracts were combined.
[0056] 2. Enrichment and purification of solid-phase extraction cartridges: first activate the Oasis HLB (3cc / 60mg) solid-phase extraction cartridges with 4mL methanol and 4mL water, then accurately pipette 600μL extract solution for loading, add 4mL water for rinsing , and then eluted with 3 mL of eluent (0.1% formic acid in methanol),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com