Nano-enzyme catalysis assisted ratio type colorimetric heparin detection method
A ratio-type, nano-enzyme technology, applied in the field of analytical chemistry, can solve the problems of complex detection operation steps, susceptibility to interference from external factors, complicated detection process, etc., and achieve ultra-sensitive and specific quantitative detection with simple experimental steps , the effect of reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
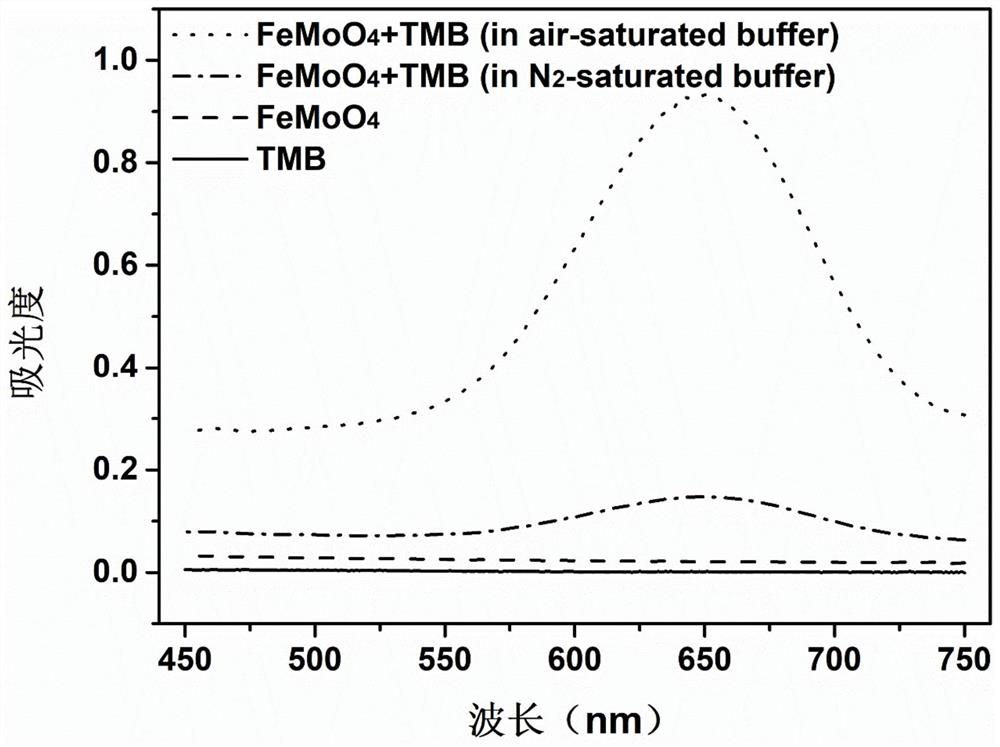
[0037] FeMoO 4 Nanorods exhibit oxidase-like activity and catalyze the oxidation of colorless TMB to generate blue TMBox
[0038] (1) Add 900 μL of 0.1M phosphate buffer (pH 7.0) to a 1.5mL centrifuge tube and shake well;
[0039] (2) Add 50 μL of 1 mg / mL FeMoO to the above buffer solution 4 Nanorod aqueous solution and 50 μL 5mMTMB solution (prepared with ethanol), ensure that the total volume of the solution is 1.0mL, and react for 1-10min;
[0040] (3) Measure the light absorption signal of the above solution with an ultraviolet-visible spectrometer, and obtain the absorbance value at 652 nm.
[0041] The result is as figure 1 as shown, figure 1 Medium TMB substrates cannot be directly oxidized by dissolved oxygen, and low concentrations of FeMoO 4 The aqueous solution (50 μg / mL) also has no background color. However, adding FeMoO to a TMB solution containing dissolved oxygen 4 After the nanorods, an obvious color reaction occurs quickly, and an obvious visible absor...
Embodiment 2
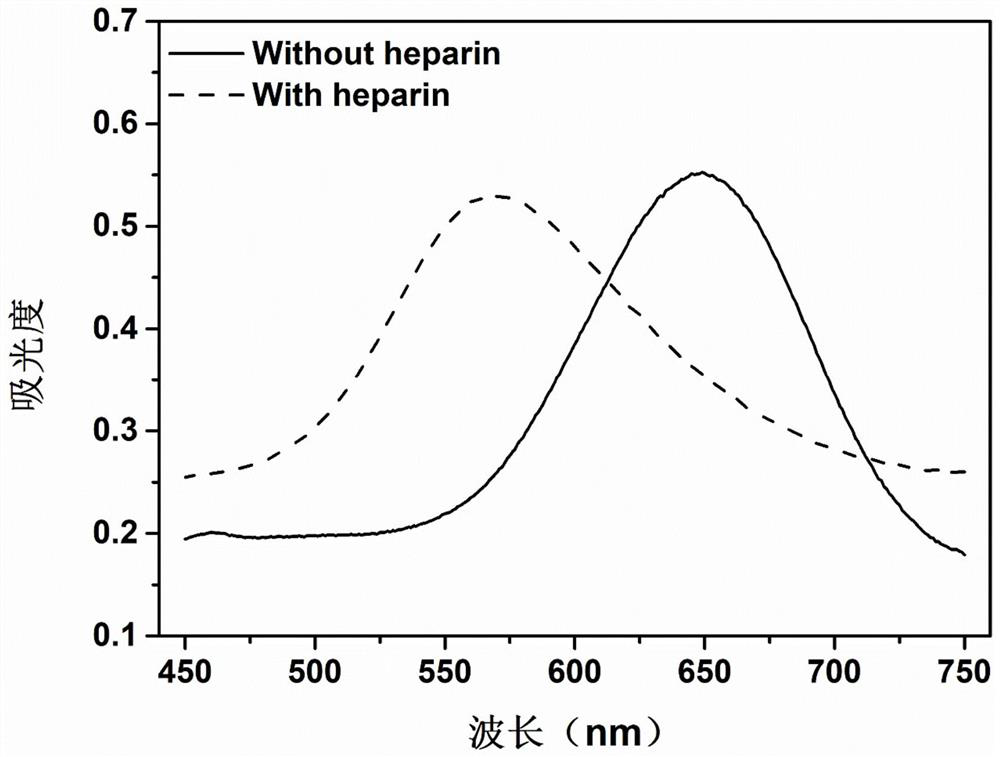
[0043] Heparin induces the aggregation of TMBox and affects the color development of the solution
[0044] (1) Add 900 μL of 0.1M phosphate buffer (pH 7.0) to a 1.5mL centrifuge tube and shake well;
[0045] (2) Add 50 μL of 1 mg / mL FeMoO to the above buffer solution 4 Nanorod aqueous solution and 25 μL 5mMTMB solution (prepared with ethanol), react for 5 minutes;
[0046] (3) Add 3 μg / mL heparin solution to the above solution and react for 2 minutes;
[0047] (4) Measure the absorbance signal of the above-mentioned solution with a UV-visible spectrometer, and obtain the absorbance values at 652nm and 565nm.
[0048] The result is as figure 2 shown, since the FeMoO 4 Under the catalysis of nanorods, TMB is oxidized into TMBox, making FeMoO 4 +TMB solution turns blue. When heparin was added (3.0μg / mL, equivalent to 0.51U / mL or 0.486μM), the solution turned purple, and a new obvious signal appeared around 565nm, while the absorbance at 652nm decreased. Furthermore, onl...
Embodiment 3
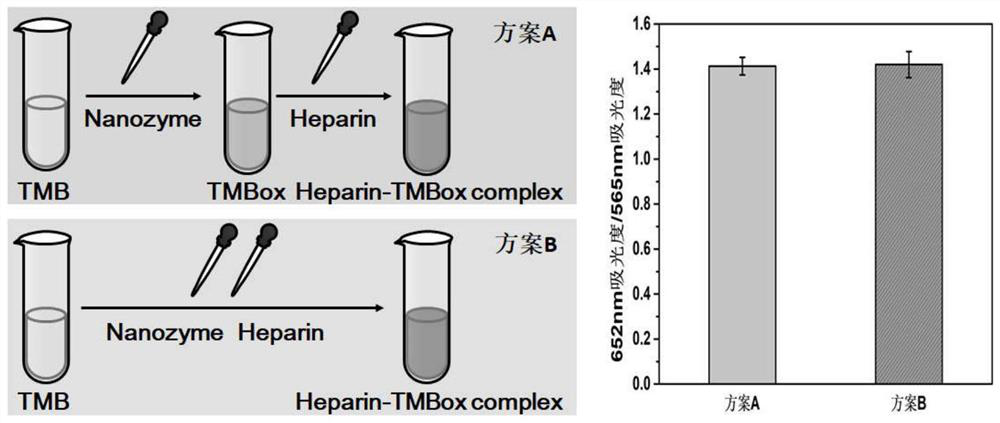
[0050] The effect of the adding order of heparin on the detection system
[0051] In this assay, the analyte heparin was added in two ways ( image 3 ): One is to add after TMB is completely catalyzed and oxidized into blue TMBox species, forming a two-step detection method; the other is to add TMB at the same time, forming a one-pot detection method. In order to check whether the two methods of operation will cause differences in the detection results, the detection results of the same heparin concentration (1.0 μg / mL) by different addition methods were compared. The results are as follows: image 3 shown. It was found that the above two addition methods had no significant difference in the detection of heparin. In order to save detection time, a one-pot method was optimally used for the detection of heparin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com