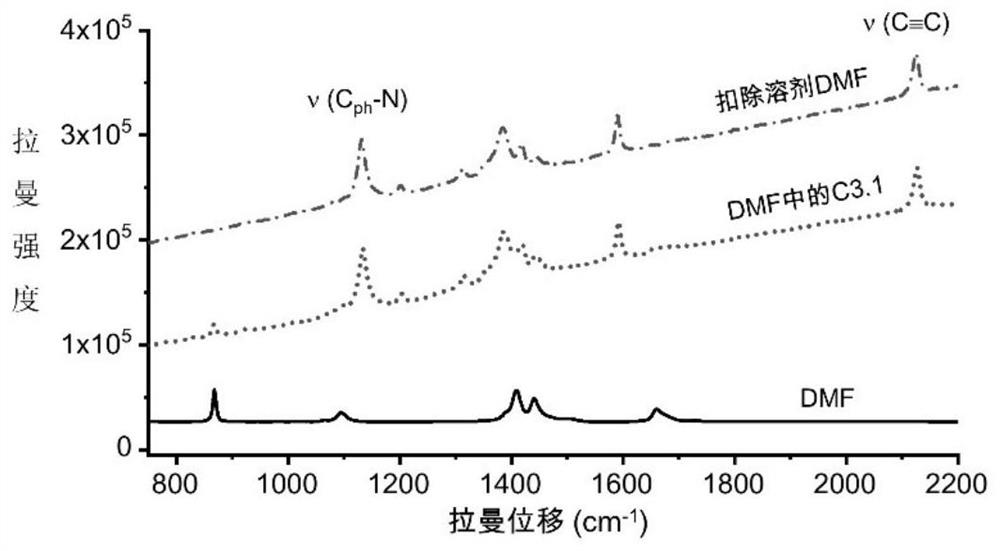
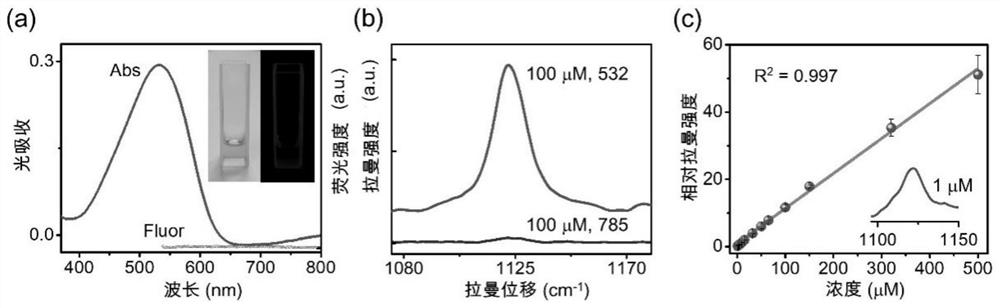
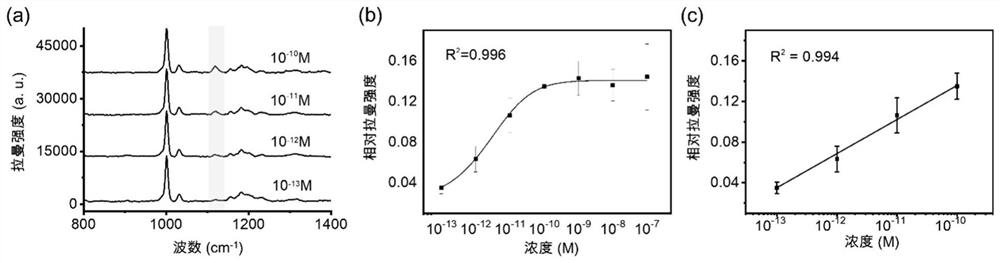
Azo aromatic compound, application thereof and reagent for enhancing Raman scattering signal
A technology of aromatic compounds and compounds, applied in the field of analytical chemistry, can solve problems such as interference with Raman signal detection, failure to provide Raman frequency adjustability, Raman signal cannot be accurately detected, etc., and achieve the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0099] Preparation Example 1: Synthesis of Group A Compounds (Core Groups are Diacetylenes)
[0100]
[0101] Add 3mL chloroform and 1mL of 1,4-dioxane to the 25mL single-neck flask, then weigh 3mmol of phenylacetylene and 1mmol of 4-ethynyl methyl benzoate and dissolve therein, add 0.05mmol of copper powder and 0.2 mmol tetramethylethylenediamine. The mixed system was placed at 50 °C and stirred for 12 h. Subsequently, 20 mL of dichloromethane was added, washed successively with saturated ammonium chloride, distilled water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. After column chromatography, a yellow solid product A1.1. was obtained (yield 65 %) 1 H NMR (400MHz, CDCl 3 )δ7.99(d,J=8.3Hz,2H),7.55(d,J=8.3Hz,2H),7.39-7.30(d,J=8.3Hz,2H),6.65-6.54(d,J=8.3 Hz,2H),3.92(s,3H). 13 C NMR (101MHz, CDCl 3 )δ166.5,147.9,134.2,132.2,129.9,129.5,127.0,114.6,110.2,84.5,79.8,77.5,71.8,52.4.HRMS(ESI):calcd for C...
preparation example 2
[0106] Preparation Example 2: Synthesis of Group B Compounds (the core group is triacetylene)
[0107]
[0108] 9mL of chloroform and 3mL of 1,4-dioxane were added to a 50mL single-neck flask, followed by weighing trimethylsilylacetylene (15mmol, 3eq) and methyl 4-ethynylbenzoate (5mmol, 1eq) to dissolve To this, copper powder (0.25 mmol, 0.05 eq) and tetramethylethylenediamine (1 mmol, 0.2 eq) were added. The mixed system was placed at 50 °C and stirred for 12 h. Subsequently, 40 mL of dichloromethane was added, washed successively with saturated ammonium chloride, distilled water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. After column chromatography, a pale yellow solid product 1 was obtained (yield 64%) . 1 H NMR (400MHz, Chloroform-d) δ8.03–7.91(m, 2H), 7.53(d, J=8.4Hz, 2H), 3.91(s, 3H), 0.23(s, 9H). 13 C NMR (101MHz, CDCl 3 )δ166.3,132.6,130.4,129.5,126.1,92.5,87.4,76.8,75.6,52.4,0.4.HRMS(ESI)...
preparation example 3
[0118] Preparation Example 3: Synthesis of Group C Compounds (the core group is tetraynes)
[0119]
[0120] Add 9mL chloroform and 3mL of 1,4-dioxane to the 50mL single-necked bottle, then weigh trimethylsilyl acetylene (15mmol) and 4-ethynylaniline (5mmol) and dissolve in it, add copper powder ( 0.25 mmol) and tetramethylethylenediamine (1 mmol). The mixed system was placed at 50 °C and stirred for 12 h. Subsequently, 40 mL of dichloromethane was added, washed with saturated ammonium chloride, distilled water and saturated brine successively, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. After column chromatography, a brown-yellow solid product 6 was obtained (yield 60%) . 1 H NMR (400MHz, Chloroform-d)δ7.28(d,J=8.4Hz,2H),6.55(d,J=8.4Hz,2H),3.90(s,2H),0.21(s,9H). 13 C NMR (101MHz, CDCl 3 )δ147.7,134.3,114.6,110.1,89.5,88.5,78.1,72.4,0.3.HRMS(ESI):calcd for C 13 H 16 NSi + [M+H] + 214.10465, found214.10468.
[0121]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com