Blood flow guiding dense net stent
A dense mesh and blood flow technology, applied in the field of medical devices, can solve problems affecting product safety and effectiveness, coating distal vascular embolism, poor blood compatibility, etc., to ensure safety and effectiveness, prevent arterial occlusion, and reduce costs. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
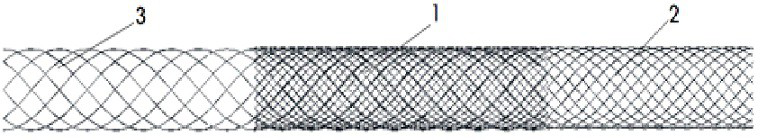
[0030] like figure 1 As shown, the present invention provides a blood flow guiding dense mesh support, including a network management support formed by interpenetrating a plurality of unit networks; the network management support is an independent network management support formed by interpenetrating three independent network management supports; as figure 2 As shown, the three independent network management supports are respectively a wire mesh support, a developing wire mesh support, and an antithrombotic polymer fiber network support; the three independent network management supports can form independent network management supports with each other. The wire diameter of the wire mesh tube stent is 0.05mm; the surface area coverage ratio of the wire and the antithrombotic polymer fiber is 8:1; the antithrombotic polymer fiber is a polyurethane fiber containing phosphorylcholine with a diameter of 0.02mm.
Embodiment 2
[0032] like figure 1 As shown, the present invention provides a blood flow directing dense mesh support, which includes a network management support formed by interpenetrating a plurality of unit networks; the network management support is an independent network management support formed by interpenetrating four independent network management supports; The four independent network management supports are respectively metal wire mesh support, developing wire mesh support, antithrombotic polymer fiber network support, and polymer fiber network support for promoting endothelialization of vascular cells; the four independent network support are mutually Between any 2 or more can constitute an independent network management support. The four independent network management supports penetrate each other to form an integral independent network management support. like image 3 and 4 As shown, the diameter range of the metal wire is 0.15mm, and the diameter of the polymer fiber is 0...
Embodiment 3
[0034] like figure 1 As shown, the present invention provides a blood flow directing dense mesh stent, which includes a network tube stent formed by interpenetrating a plurality of unit networks; The net tube support, the developing wire net tube bracket, the polyester fiber net tube bracket containing phosphorylcholine, and the polyurethane fiber net tube bracket containing vascular endothelial growth factor VEGF are composed of five independent net tube brackets; the five independent net tube brackets are formed by interpenetrating each other An integral independent network management support; any two or more of the five independent network management supports may form an independent network management support. like Figure 5 As shown, the wire mesh pipe support with a diameter of 0.02mm and the wire mesh pipe support of 0.05mm penetrate each other and can also independently form a separate network pipe support; Figure 6 As shown, the antithrombotic polymer fiber mesh ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com