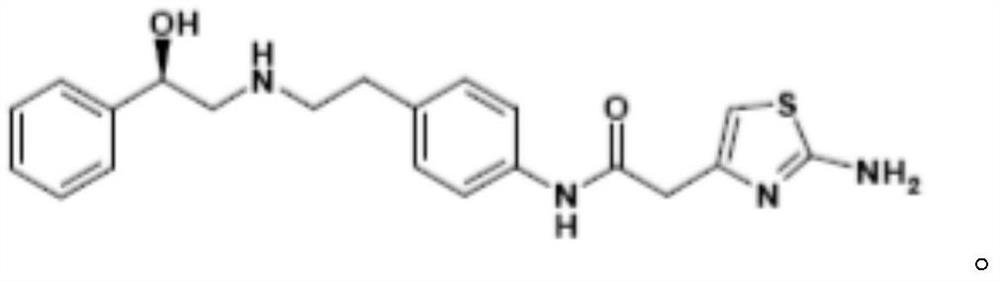
Mirabegron sustained-release composition as well as preparation method and application thereof
A technology for a composition and a sustained-release tablet, which is applied in the field of mirabegron sustained-release composition and its preparation, can solve the problems of cumbersome granulation operation, difficulty in the content uniformity of active ingredients, etc. Particle size requirements, the effect suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
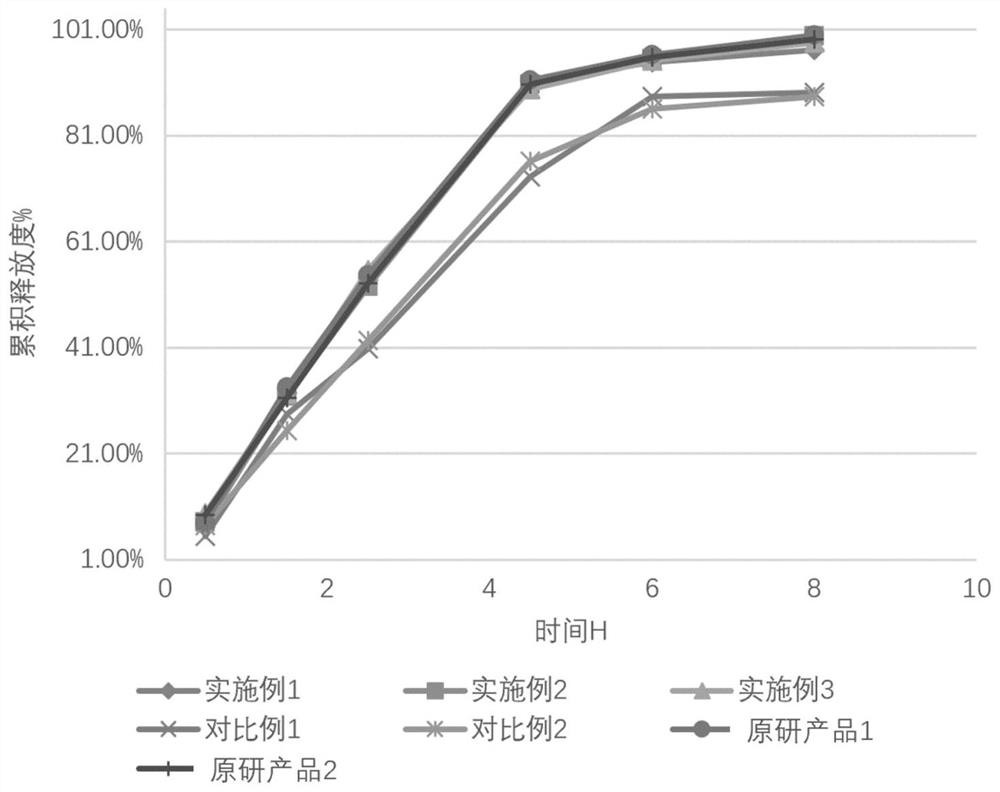
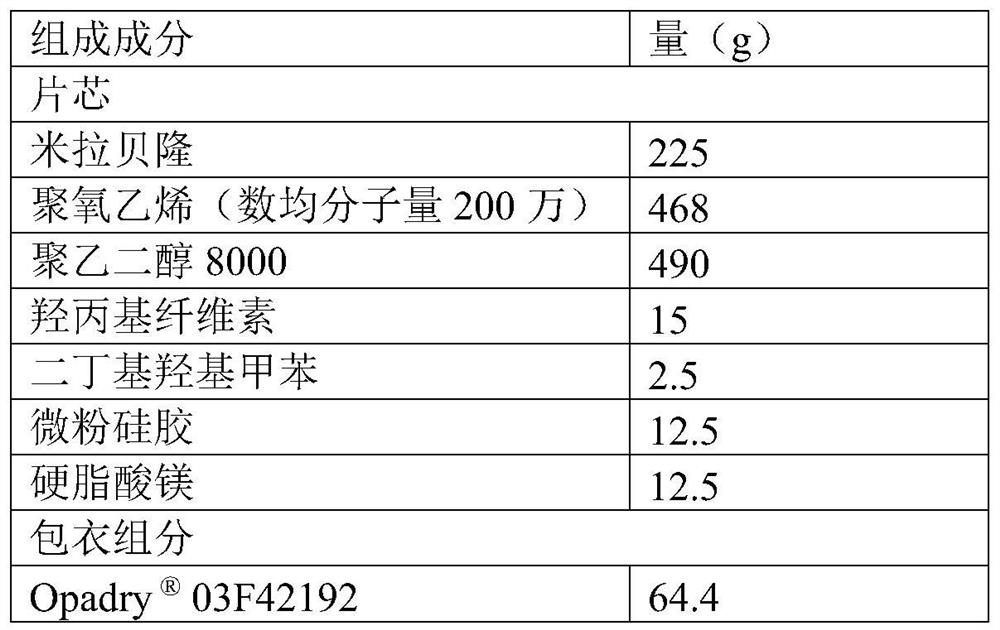
[0068] The formulation of Mirabegron extended-release tablets:
[0069]
[0070] The preparation method of Mirabegron sustained-release tablet, comprises the steps:
[0071] (1) Pass the hydroxypropyl cellulose, micronized silica gel and magnesium stearate through a 60-mesh sieve for later use.
[0072] (2) Under nitrogen protection, heat 468g of polyoxyethylene and 490g of polyethylene glycol 8000 in a water bath (60°C-80°C), and stir until melting.
[0073] (3) Add 225 g of mirabegron and 2.5 g of dibutyl hydroxytoluene to the above co-melt, stir for 10 min, and mix well. Allow to cool to room temperature and become solid.
[0074] (4) Use a water-cooled pulverizer (installed with a 20-mesh screen) to pulverize the above solid, use an ice-water bath to control the pulverization temperature below 20°C, measure the particle size after the pulverization, D 90 is 506 μm.
[0075] (5) Mix the pulverized material and 15 g of hydroxypropyl cellulose in a mixer for 10 minutes...
Embodiment 2
[0081] The formulation of Mirabegron extended-release tablets:
[0082]
[0083] The preparation method of Mirabegron sustained-release tablet, comprises the steps:
[0084] (1) Pass the hydroxypropyl cellulose, micronized silica gel and magnesium stearate through a 60-mesh sieve for later use.
[0085] (2) Under nitrogen protection, heat 434g of polyoxyethylene and 544g of polyethylene glycol 8000 in a water bath (60°C-80°C), and stir until melting.
[0086] (3) Add 225 g of mirabegron and 2.5 g of dibutyl hydroxytoluene to the above co-melt, stir for 10 min, and mix well. Allow to cool to room temperature and become solid.
[0087] (4) Use a water-cooled pulverizer (installed with a 20-mesh screen) to pulverize the above solid, use an ice-water bath to control the pulverization temperature below 20°C, measure the particle size after the pulverization, D 90 is 500 μm.
[0088] (5) Mix the pulverized material and 20 g of hydroxypropyl cellulose in a mixer for 10 minutes...
Embodiment 3
[0094] The formulation of Mirabegron extended-release tablets:
[0095]
[0096] The preparation method of Mirabegron sustained-release tablet, comprises the steps:
[0097] (1) Pass the hydroxypropyl cellulose, micronized silica gel and magnesium stearate through a 60-mesh sieve for later use.
[0098] (2) Under nitrogen protection, heat 400g of polyoxyethylene and 568g of polyethylene glycol 8000 in a water bath (60°C-80°C), and stir until melting.
[0099] (3) Add 225 g of mirabegron and 2.5 g of dibutyl hydroxytoluene to the above co-melt, stir for 10 min, and mix well. Allow to cool to room temperature and become solid.
[0100] (4) Use a water-cooled pulverizer (installed with a 20-mesh screen) to pulverize the above solid, use an ice-water bath to control the pulverization temperature below 20°C, measure the particle size after the pulverization, D 90 is 518 μm.
[0101] (5) Mix the pulverized material and 35 g of hydroxypropyl cellulose in a mixer for 10 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com