Organic sulfur hydrolysis catalyst applicable to Claus process as well as preparation method and application of organic sulfur hydrolysis catalyst
A hydrolysis catalyst and organosulfur technology, applied in the field of organosulfur hydrolysis catalyst and its preparation, can solve problems such as high unfavorable Claus reaction, and achieve the effects of high-efficiency hydrolysis, strong tunability of components and structures, and ultra-low emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
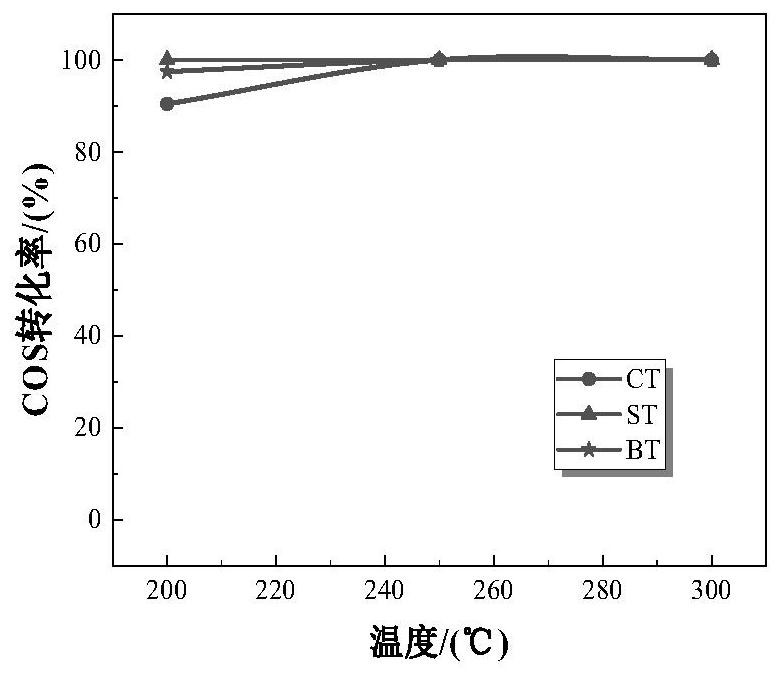
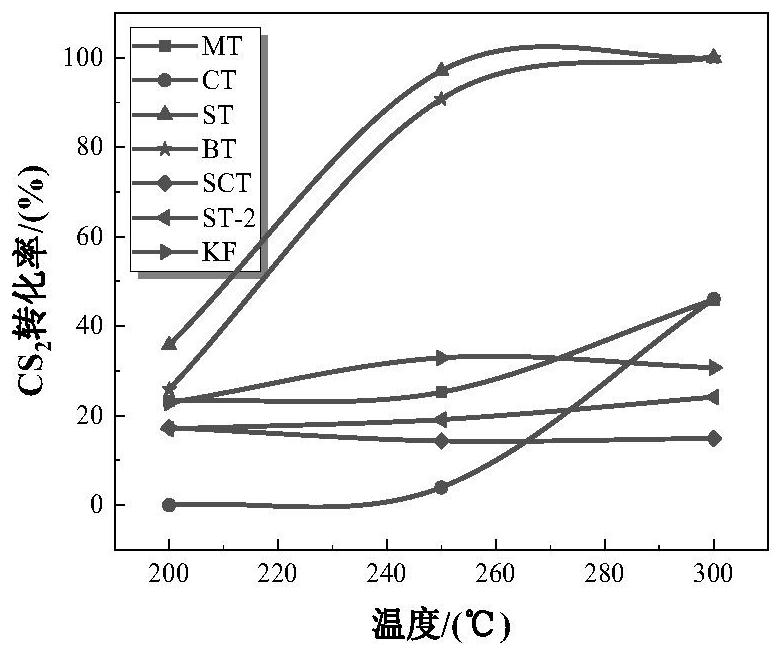
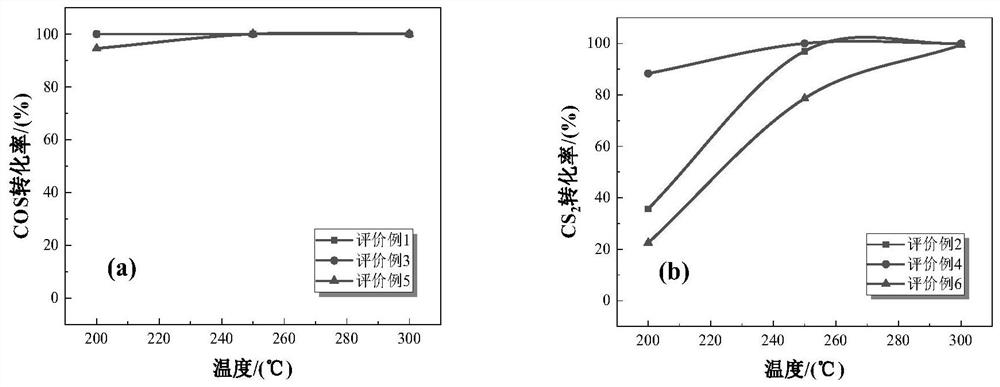
[0042] 7.692g of magnesium nitrate hexahydrate was dissolved in 100mL of deionized water, under vigorous stirring, was added sequentially 8.88mL tetraisopropyl titanate, 2.4 g of sodium hydroxide, an alkali metal compound, stirred for 1h. The resulting solution was transferred to a 180mL capacity of the hydrothermal reaction vessel, the reaction oven 24h 200 ℃. Taken sample of the solution with deionized water and ethanol by centrifugation, washed three times and placed in an oven at 120 deg.] C and dried 12h. Powder sample was taken, placed in a muffle furnace 650 ℃ calcined 6h, heating rate 5 ℃ / min, the resultant sample was referred to as MT.
Embodiment 2
[0044] 7.085g of calcium nitrate tetrahydrate was dissolved in 100mL of deionized water, under vigorous stirring, was added sequentially 8.88mL tetraisopropyl titanate, 2.4 g of sodium hydroxide, an alkali metal compound, stirred for 1h. The resulting solution was transferred to a 180mL capacity of the hydrothermal reaction vessel, the reaction oven 24h 200 ℃. Taken sample of the solution with deionized water and ethanol by centrifugation, washed three times and placed in an oven at 120 deg.] C and dried 12h. Powder sample was taken, placed in a muffle furnace 650 ℃ calcined 6h, heating rate 5 ℃ / min, the resultant sample was referred to as CT.
Embodiment 3
[0046] The strontium nitrate was dissolved in 6.349g 100mL deionized water, under vigorous stirring, was added sequentially 8.88mL tetraisopropyl titanate, 2.4 g of sodium hydroxide, an alkali metal compound, stirred for 1h. The resulting solution was transferred to a 180mL capacity of the hydrothermal reaction vessel, the reaction oven 24h 200 ℃. Taken sample of the solution with deionized water and ethanol by centrifugation, washed three times and placed in an oven at 120 deg.] C and dried 12h. Powder sample was taken, placed in a muffle furnace 650 ℃ calcined 6h, heating rate 5 ℃ / min, the resultant sample was referred to as ST.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com