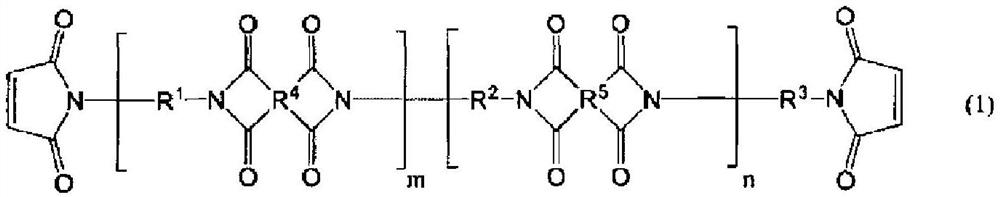
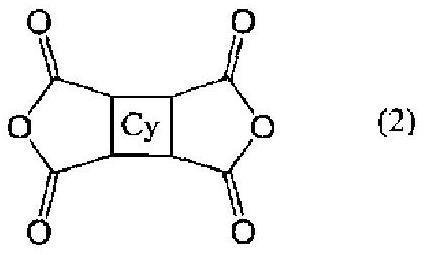
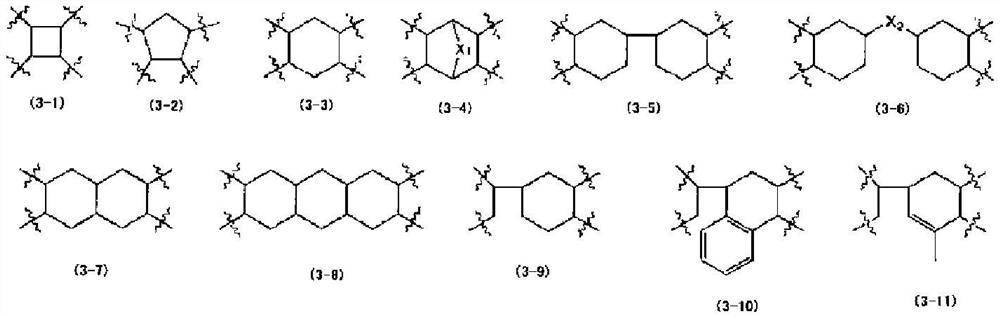
Bismaleimide compound, photosensitive resin composition using same, cured product from said photosensitive resin composition, and semiconductor element
A bismaleimide and compound technology, applied in the field of bismaleimide compounds, can solve the problems of insufficient patterning performance and wafer design limitations, and achieve small tensile elastic modulus and adhesion excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0135] Hereinafter, although this invention is demonstrated more concretely based on an Example and a comparative example, this invention is not limited to a following example. In addition, the patterning performance evaluation and mechanical property evaluation in each Example and a comparative example were performed as follows.
[0136] The measurement conditions of the molecular weight are as follows.
[0137] Model: GPC TOSOH HLC-8220GPC
[0138] Column: Super HZM-N
[0139] Eluent: THF (tetrahydrofuran); 0.35ml / min, 40°C
[0140] Detector: RI (Differential Refractometer)
[0141] Molecular weight standard: polystyrene
Synthetic example 1(I-1
[0143] 110 g of toluene and 36 g of N-methylpyrrolidone were charged into a 500 ml round bottom flask equipped with a stirring bar coated with the fluororesin. Next, 88.0 g (0.16 mol) of PRIAMINE 1074 (manufactured by Croda Japan Co., Ltd.) was added, and then, 15.8 g (0.16 mol) of methanesulfonic anhydride was slowly added to form a salt. Stir roughly for 10 minutes to mix, then slowly add 5-(2,5-dioxotetrahydrofuryl)-3-methyl-3-cyclohexene-1,2-dicarboxylic dianhydride ( 21.8 g, 0.08 mol). Attach a Dean-Stark separator and condenser to the flask. The mixture was heated to reflux for 6 hours to form the amine terminated imide. The theoretical amount of water formed from this condensation is obtained by this time. The reaction mixture was cooled below room temperature, and 19.4 g (0.20 mol) of maleic anhydride was added to the flask. The mixture was refluxed for 8 hours to obtain the expected amount of water formed. After cooling to room temperature, 200 ml of toluene was ...
Synthetic example 2(I-2
[0145] 110 g of toluene and 36 g of N-methylpyrrolidone were charged into a 500 ml round bottom flask equipped with a stirring bar coated with the fluororesin. Next, 90.5 g (0.17 mol) of PRIAMINE 1074 (manufactured by Croda Japan Co., Ltd.) was added, and then, 16.3 g (0.17 mol) of methanesulfonic anhydride was slowly added to form a salt. Stir roughly for 10 minutes to mix, then slowly add 1,2,4,5-cyclohexanetetracarboxylic dianhydride (18.9 g, 0.08 mol) to the stirred mixture. Attach a Dean-Stark separator and condenser to the flask. The mixture was heated to reflux for 6 hours to form the amine terminated imide. The theoretical amount of water formed from this condensation is obtained by this time. The reaction mixture was cooled below room temperature, and 19.9 g (0.20 mol) of maleic anhydride was added to the flask. The mixture was refluxed for 8 hours to obtain the expected amount of water formed. After cooling to room temperature, 200 ml of toluene was then added to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com