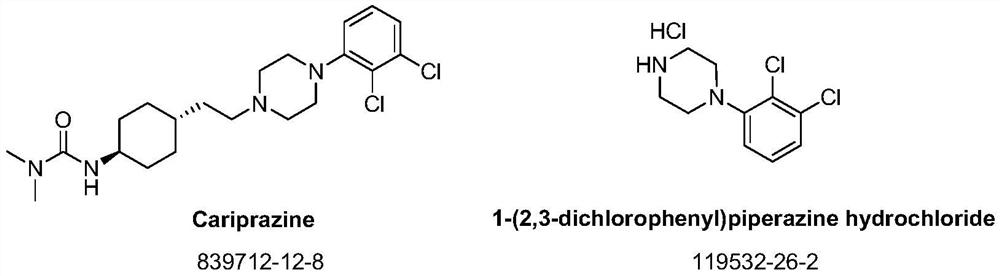
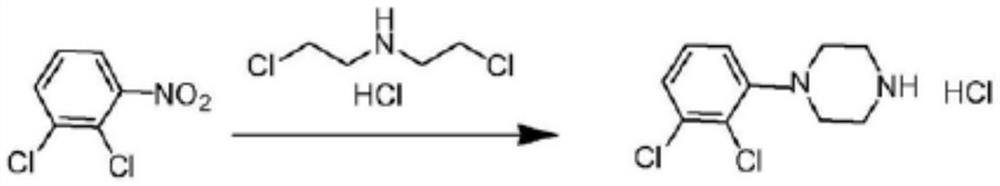
Synthesis method of cariprazine key intermediate 1-(2,3-dichlorophenyl)piperazine hydrochloride
A technology of dichlorophenyl and hydrochloride, which is applied in the field of drug synthesis, can solve the problems of no cost advantage, swelling, and low yield, and achieve good application prospects, simple equipment requirements, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
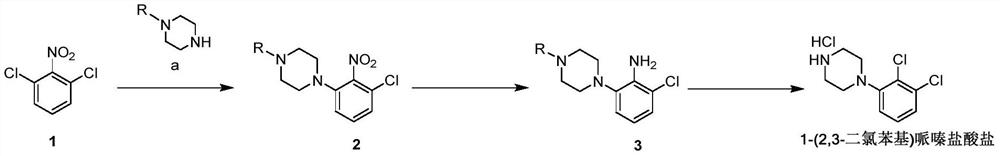
[0042] Embodiment 1: Preparation 1-(2,3-dichlorophenyl) piperazine hydrochloride
[0043] Prepare 1-(2,3-dichlorophenyl)piperazine hydrochloride according to the following reaction scheme:
[0044]
[0045] Step 1: Preparation of compound 2:
[0046]
[0047] Add potassium carbonate (43.1g, 0.313mol, 0.6eq) and diisopropylethylamine (40.4g, 0.313 mol, 0.6eq), stirred at 22°C for 5min. Add pivaloylpiperazine (88.7g, 0.521mol, 1.0eq), stir at 22°C for 6h, then raise the temperature to 52°C and stir for 2h. After the reaction, the reaction solution was poured into water (1200mL), extracted with dichloromethane (500mL×2), the organic phases were combined, water (300mL) was added, and the pH of the aqueous phase was adjusted to 5.5-6.0 with 20% citric acid. Liquid separation. The organic phase was concentrated under reduced pressure to obtain 146.0 g of compound 2 with a yield of 86%.
[0048] Step 2: Preparation of compound 3:
[0049]
[0050] To the solution of comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com