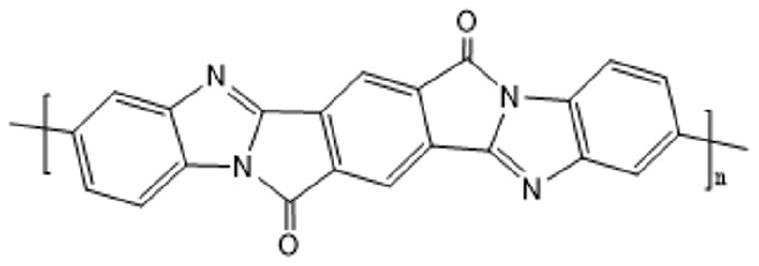
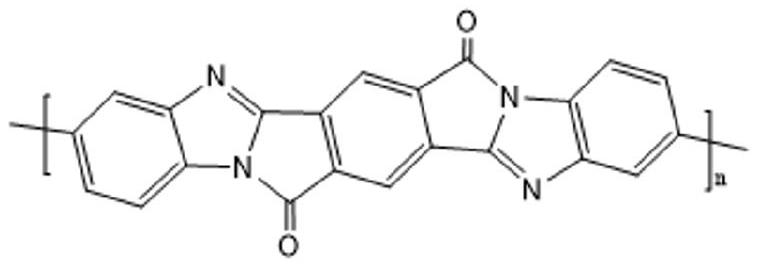
High-strength and high-modulus polypyrrolone fiber and preparation method thereof
A high-strength, high-modulus, polypyrrolidone technology, applied in the fields of fiber chemical characteristics, single-component synthetic polymer rayon, chemical post-processing of synthetic polymer rayon, etc., can solve the limitations of processability, high boiling point, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A second aspect of the present invention provides a method for preparing the above-mentioned high-strength and high-modulus polypyrrole fiber, which includes the following steps:
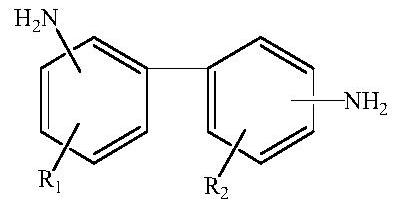
[0030] (1) Preparation of raw material monomer: acetic anhydride is dissolved in organic solvent A to obtain reaction material A, then said reaction material A is added in the organic solvent B solution of substituted biphenyl diamine, and Reacting at a reaction temperature of °C for 2 to 6 hours to obtain an intermediate crude product; adding calcium oxide to the intermediate crude product for precipitation, filtering, and concentrating to obtain a crude product; then recrystallizing the crude product to obtain the raw material monomer;
[0031] (2) Synthesis of the intermediate polyacetamidoamic acid: adding the raw material monomers into the reactor to carry out condensation reaction with the dibasic acid anhydride in the spinning solvent to obtain the polyacetamidoamic acid solution;
[0...
Embodiment 1
[0063] (1) Preparation of raw materials: 100 grams (466.7 mmol) of 3,3'-diaminobenzidine was placed in a reactor and dissolved in 567 grams of THF to prepare a solution with a concentration of 15%, and then in the above 3,3'- In the THF solution of diaminobenzidine (M=214.27), 952.9 grams (2*466.7mmol acetic anhydride (M=102.09), weight 95.29 grams) of 10% acetic anhydride THF solution was added dropwise at a speed of 3-5g / min, Control the reaction temperature in the range of -5~0°C for 4 hours, then add 22.6g (466.7mmol) of dry calcium oxide (M=56.077) to react with acetic acid to form calcium acetate precipitate, which is precipitated from the THF solution; Calcium acetate, and the filtrate was concentrated by rotary evaporation to obtain 136 grams of crude product (theoretical yield is 139.2 grams) of 3,3'-diamino-4,4'-diacetamidobiphenyl (M=298.27); ,3'-diamino-4,4'-diacetylaminobiphenyl crude product was added ethanol / THF solvent with a volume ratio of 1 / 1, and recrystall...
Embodiment 2
[0067] (1) Preparation of raw materials: 100 grams (466.7 mmol) of 3,3'-diaminobenzidine was placed in a reactor and dissolved in 567 grams of THF to prepare a solution with a concentration of 15%, and then in the above 3,3'- In the THF solution of diaminobenzidine (M=214.27), 952.9 grams (2*466.7mmol acetic anhydride (M=102.09), weight 95.29 grams) of 10% acetic anhydride THF solution was added dropwise at a speed of 3-5g / min, Control the reaction temperature in the range of -5~0°C for 4 hours, then add 22.6g (466.7mmol) of dry calcium oxide (M=56.077) to react with acetic acid to form calcium acetate precipitate, which is precipitated from the THF solution; Calcium acetate, and the filtrate was concentrated by rotary evaporation to obtain 136 grams of crude product (theoretical yield is 139.2 grams) of 3,3'-diamino-4,4'-diacetamidobiphenyl (M=298.27); ,3'-diamino-4,4'-diacetylaminobiphenyl crude product was added ethanol / THF solvent with a volume ratio of 1 / 1, and recrystall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com