Application of neural stem cells combined with umbilical cord mesenchymal stem cells in spinal cord injury
A technology for neural stem cells and spinal cord injury, which is applied in the direction of nervous system cells, animal cells, and nervous system diseases to achieve the effect of promoting tissue repair, reducing the area, and promoting the repair of nerve damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Ethical statement:
[0034] The collection of all human specimens and the implementation of animal experiments were approved by the Experimental Ethics Committee of Hebei Medical University (guarantee number: 20190505). Experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals.
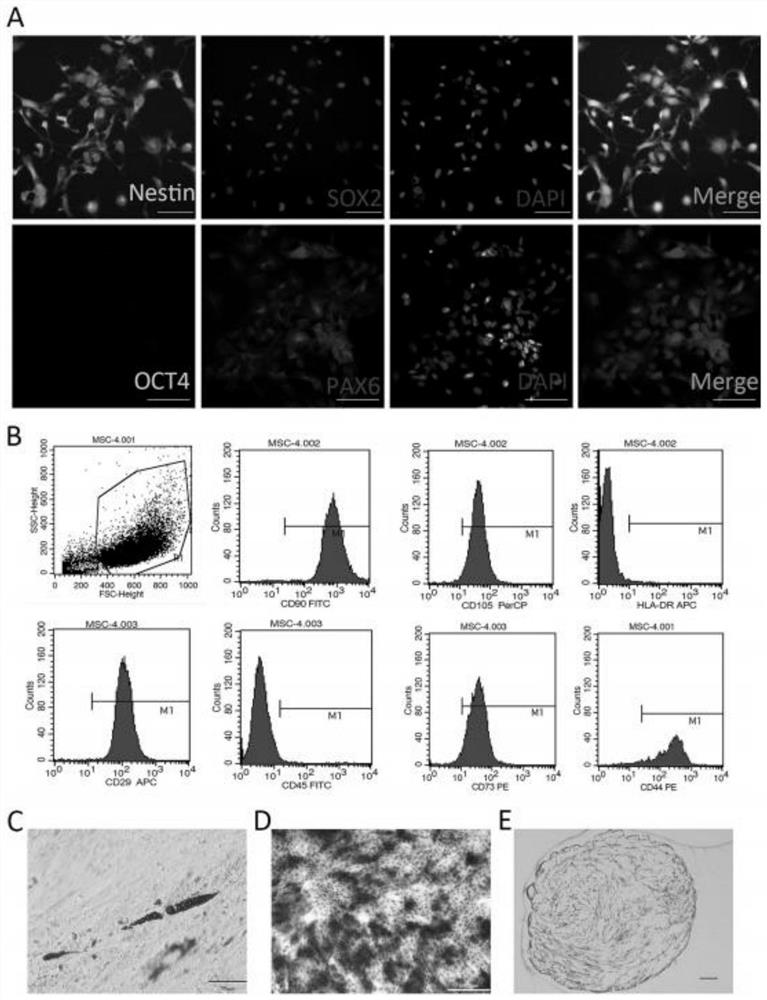
[0035] Obtain iPSC-derived neural stem cells
[0036] hhiPSCs were generated from dermal fibroblasts using a protocol previously described in a study in our laboratory (cell line: HEBHMUi002-a). Briefly, following the manufacturer's guidelines, use Cytotune TM - iPS 2.0 Sendai Reprogramming Kit (Thermo Fisher Scientific, Waltham, MA, USA), to reprogram fibroblasts at passage 4 into hiPSCs. On day 0 of transduction, 4 reprogramming factors were added to fibroblasts, and the medium was changed after 24 hours to remove virus. On day 7 post-transduction, cells were transferred at the recommended density into 6-well plates coated with Geltrex hESC ce...
Embodiment 2
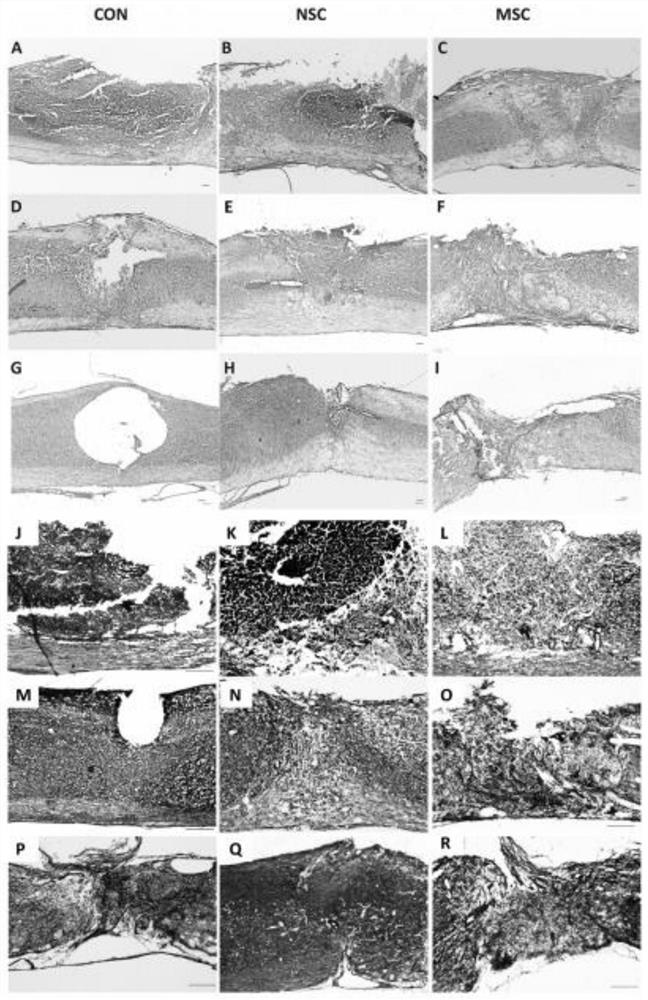
[0041] Spinal cord injury model establishment
[0042] Eight-week-old BALB / c female immunodeficiency female nude mice (19-22g, n=54) were anesthetized by intraperitoneal injection of 1% sodium pentobarbital (50mg / kg, intraperitoneal injection), Wuhan, Hubei Xinrunde Chemical Co., Ltd. company. China). A laminectomy was performed at the 10th thoracic vertebra to expose the dorsal surface of the spinal dura mater. Spinal cord contusion was induced at T10 level with a Zhongshi impactor (Zhongshi, Beijing, China) weighing 10 g and 5 cm high.
Embodiment 3
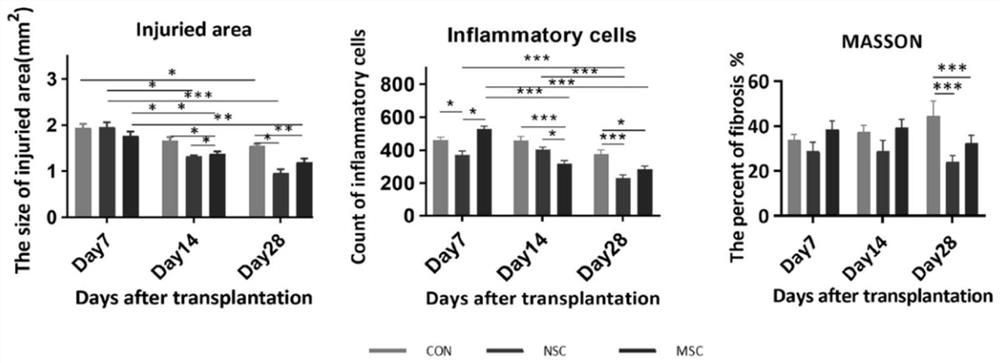
[0044] Acquisition of products for the treatment of acute spinal cord injuries in animals;
[0045] The human induced pluripotent stem cell-derived neural stem cells (NSC) combined with human umbilical cord-derived mesenchymal stem cells (MSC) are used in a ratio of 1:1, and the vehicle is PBS;
[0046] The human induced pluripotent stem cells are differentiated into NSCs in vitro through the reprogramming of human peripheral blood mononuclear cells into induced pluripotent stem cells, and the stem cells are differentiated into neural stem cells in vitro under the action of a neural inducer, and placed in a 37°C, 5% CO2 incubator middle;
[0047] In the process of differentiation into neural stem cells, the medium was changed every other day with the neural induction medium preheated at 37°C, co-cultured for seven days, and the cells were digested and passaged on the eighth day to obtain P0 NSCs.
[0048] The human umbilical cord is isolated and cultured as MSC, comprising th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com