Heptamethine cyanine small-molecular compound containing diphosphate structure and preparation method and application thereof
A small molecule compound, the technology of heptacyanine, which is applied in the field of small molecule compounds and preparation of heptacyanine, can solve the problem of not having bone-targeted accumulation and the like, and achieve the effect of preventing bone loss.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
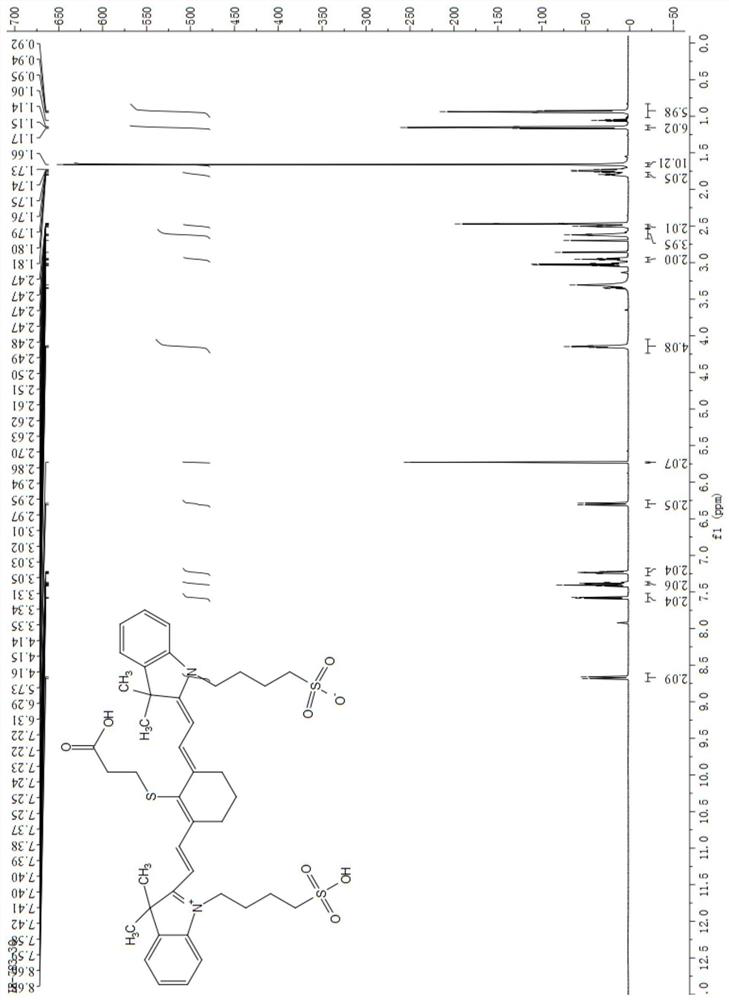
[0039] The synthesis of embodiment 1 intermediate compound (IR-3Q)
[0040]
[0041] IR-783 (982.9 mg) was charged into a 25 mL round bottom reaction vial and dissolved with 12 mL of anhydrous DMF. Add 3-mercaptopropionic acid (206.7mg) under stirring, heat up to 40°C after completion, continue to stir the reaction, and monitor the reaction by thin-layer chromatography. When thin-layer chromatography showed that there was no remaining IR-783, the heating was stopped, and the mixture was naturally cooled to room temperature. The solvent in the reaction solution was distilled off under reduced pressure to obtain a dark red viscous substance, which was purified by silica gel column chromatography as a dark green solid.
[0042] IR-3Q: 1H NMR (600MHz, DMSO-d): δ8.68, 8.66, 7.59, 7.58, 7.42, 7.41, 7.40, 7.40, 7.39, 7.38, 7.37, 7.37, 7.25, 7.25, 7.24, 7.23, 7.22, 7.22, 6.31, 6.29,5.73,4.16,4.15,4.14,3.35,3.34,3.33,3.31,3.05,3.03,3.02,3.01,2.97,2.95,2.94,2.86,2.70,2.63,2.62,2.6...
Embodiment 2
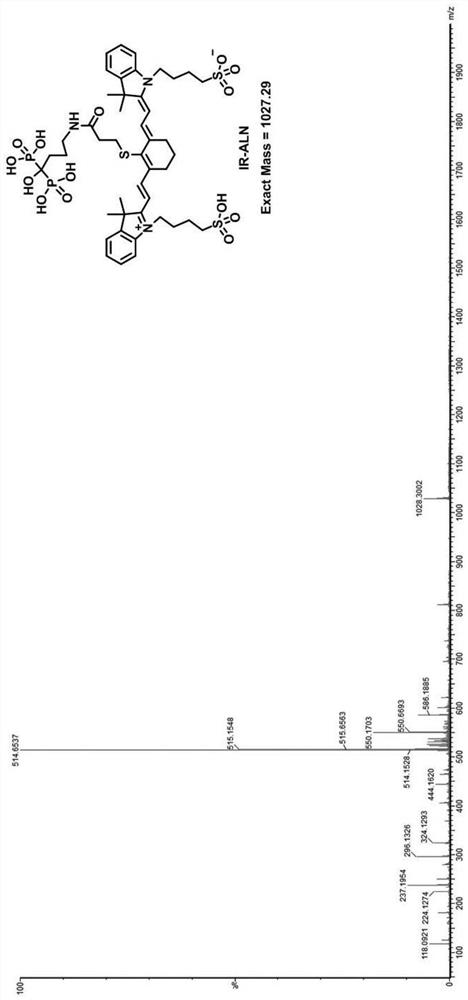
[0044] The synthesis of embodiment 2 target compound IR-ALN
[0045]
[0046] The above-prepared compound IR-3Q (150 mg) was added to a 25 mL reaction bottle, and 8 mL of dichloromethane was added. After stirring and dissolving, the condensing agent dicyclohexylcarbodiimide (DCC, 340.4 mg) and the activator N-hydroxysuccinimide (NHS, 190 mg) were added. After the addition was complete, the stirring reaction was continued at 20° C. for 12 h, and a large amount of white solid was formed. After suction filtration under reduced pressure, the filtrate was concentrated under reduced pressure to obtain the carboxyl-activated intermediate compound IR-3Q, which was carried out to the next step without further purification.
[0047] Sodium alendronate (26 mg) was dissolved in 4 mL of boric acid buffer (pH=8.4) to obtain solution a. At the same time, the intermediate IR-3Q prepared above was redissolved with anhydrous acetonitrile (2 mL) to obtain solution b. Under stirring at room...
Embodiment 3
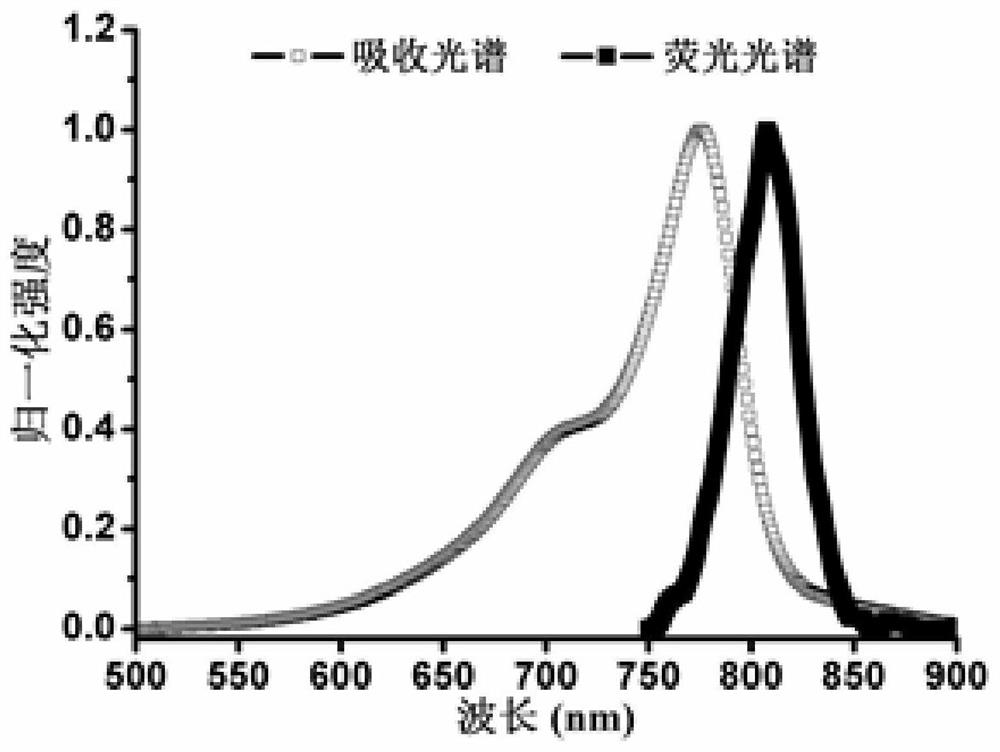
[0049] Optical performance test of embodiment 3 IR-ALN
[0050] Dissolve IR-ALN in DMSO, prepare a stock solution with a concentration of 10 mM, and store it at -20°C until use; before use, dilute with phosphate buffer (PBS, pH 7.22) to obtain a final concentration of 2 μM IR-ALN working fluid. Utilize ultraviolet-visible-near-infrared spectrometer (purple UV-3600 scanning spectrophotometer, Japan Shimadzu) to test this solution and obtain its absorption spectrum; Fluorescence spectra in the wavelength range of 750-900nm (such as image 3 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com