Synthesis process of gabapentin intermediate
A technology of gabapentin and synthesis process, which is applied in the field of synthesis process of gabapentin intermediates, can solve the problems of affecting product quality, consumption of concentrated sulfuric acid, and small environmental pollution, and achieves the effects of high reuse rate, reduced processing cost, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The technical solutions of the present invention will be clearly and completely described below in conjunction with the embodiments. Obviously, the described embodiments are part of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
[0020] In addition, the technical features involved in the different embodiments of the present invention described below may be combined with each other as long as there is no conflict with each other.
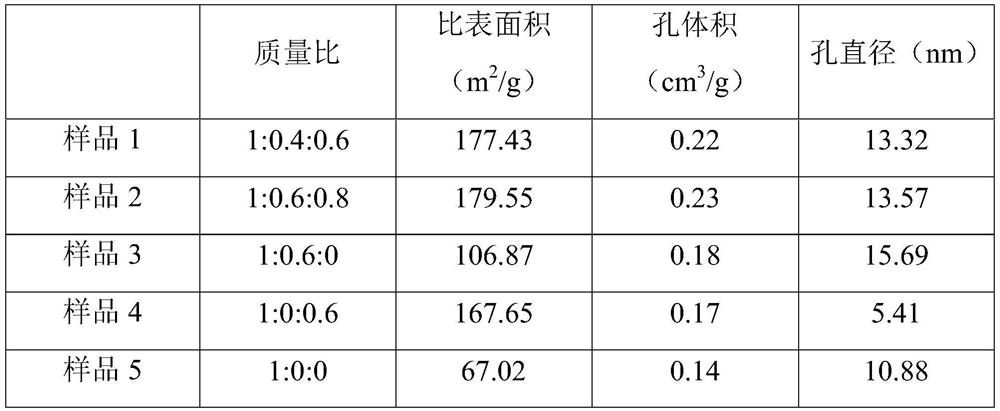
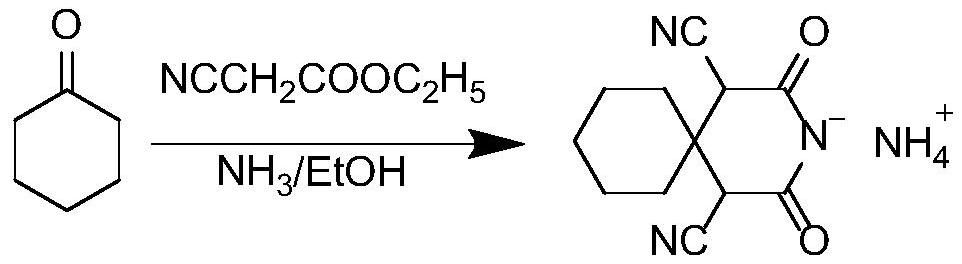
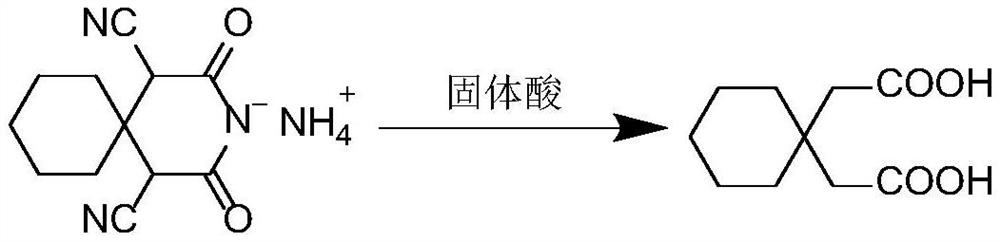
[0021] A synthesis process of a gabapentin intermediate, the intermediate being 1,1-cyclohexyl diacetic acid, the synthesis process is to first use cyclohexanone and ethyl cyanoacetate to obtain α, α'-dicyano -1,1-cyclohexyl diacetylimide ammonium salt (product I), followed by hydrolysis under the action of solid acid t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com