Preparation method of ferronickel-based nanosheet/foamed nickel oxygen evolution reaction electrode material
An oxygen evolution reaction and electrode material technology, applied in the field of electrochemistry, can solve the problems of active component shedding, complicated preparation process, performance attenuation, etc., and achieve the effect of strong binding force, simple and effective preparation process, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
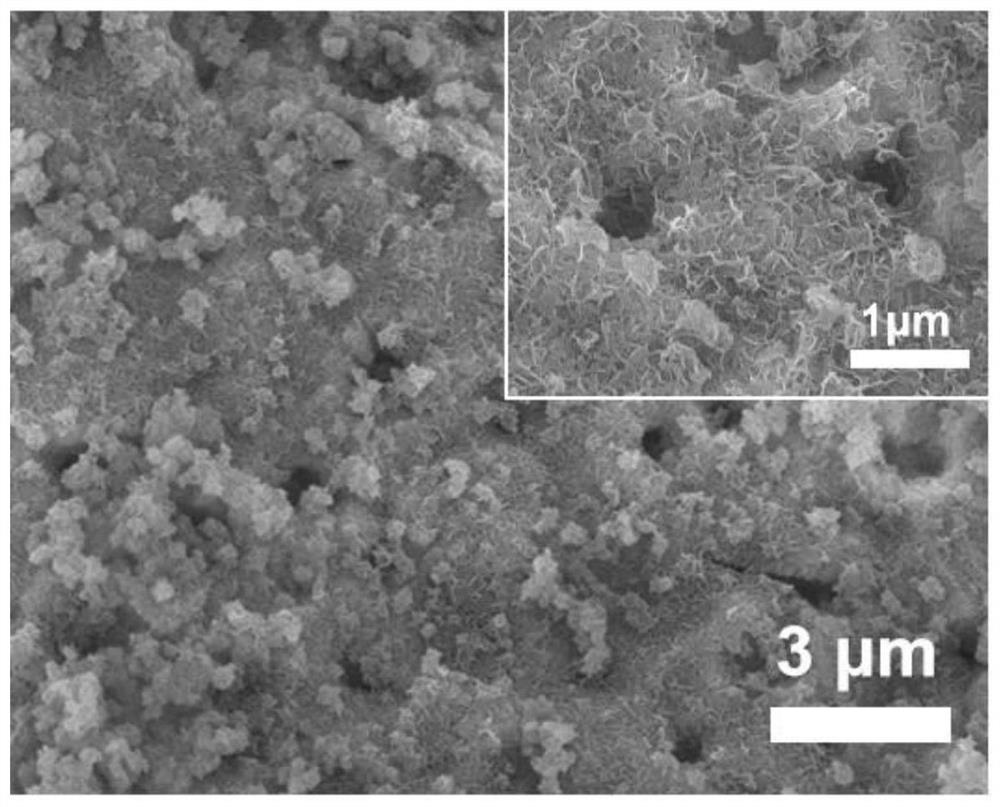
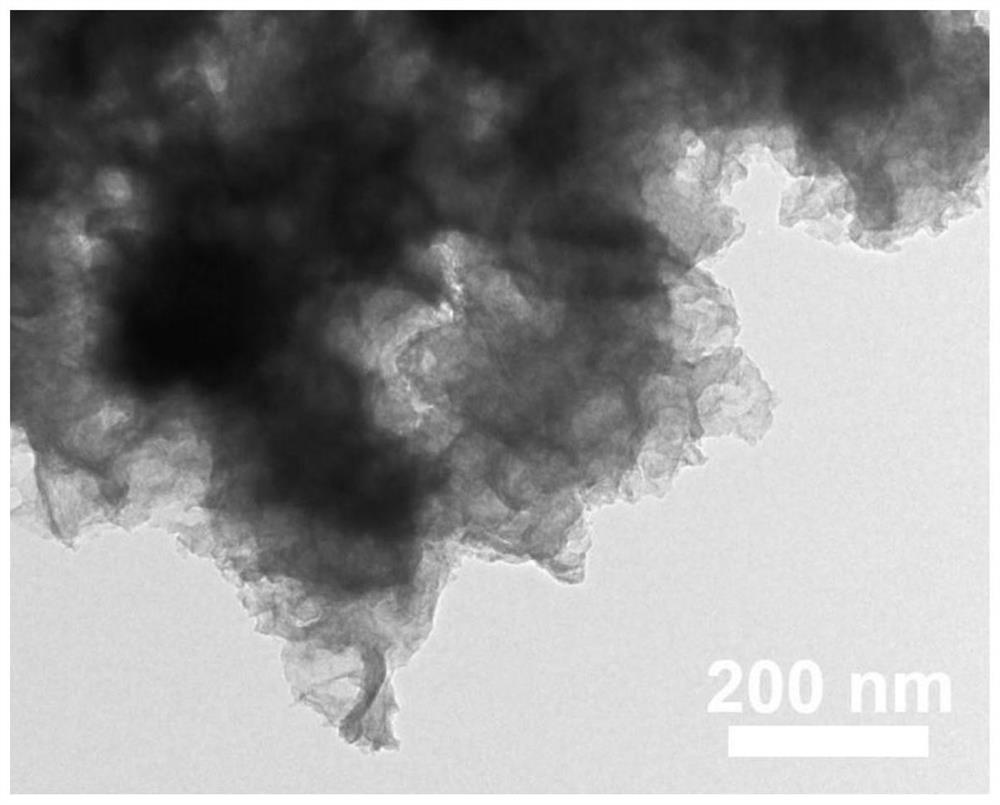
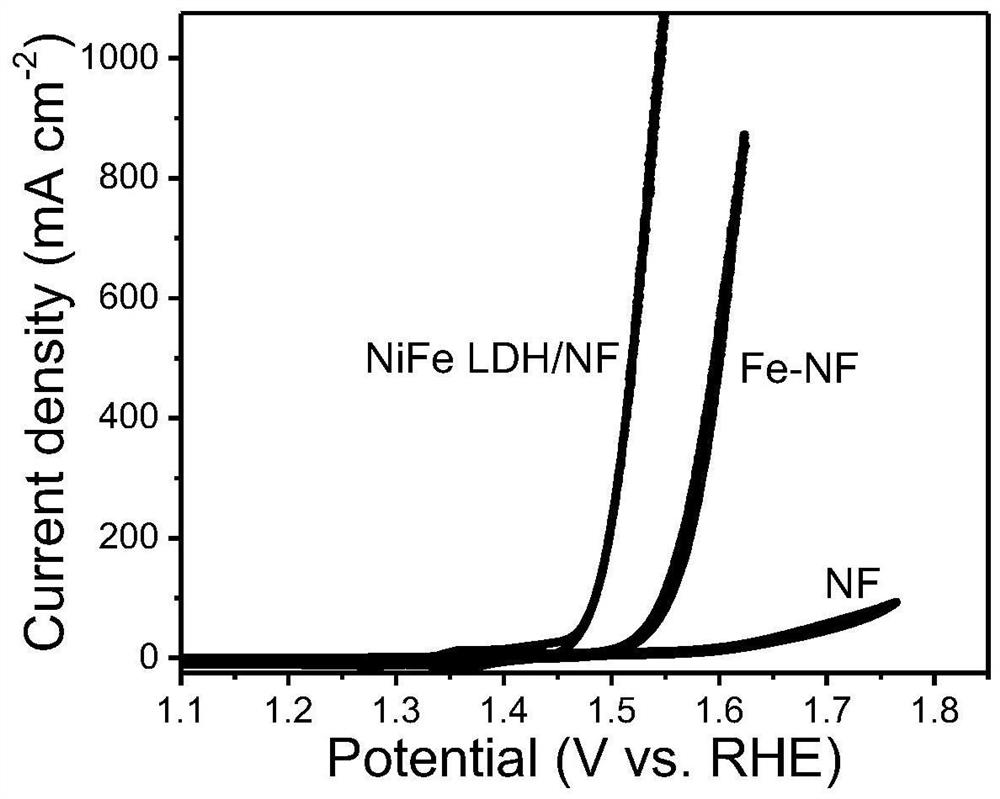
[0029] Sodium hydroxide was used to adjust the pH of the phytic acid solution to 6, wherein the concentration of phytic acid was 15 mmol / L, and the nickel foam was soaked in the above solution at room temperature for 10 min. Then the nickel foam was transferred to ferric nitrate solution and left to stand for 10 min, wherein the concentration of ferric nitrate solution was also 15mmol / L. Repeat the above process 5 times, take out the nickel foam, wash and dry. Finally, the modified nickel foam was directly used as an electrode, and the nickel-iron-based nanosheet / nickel foam oxygen evolution reaction electrode material (NiFe LDH / NF) was obtained by in-situ electrochemical activation by cyclic voltammetry, and the cycle range was 1.0V- 2.0V (vs.RHE), scan rate is 2mV / s, cycle 5 times. refer to figure 1 , nickel-iron-based nanosheets are distributed on the surface of nickel foam, with a length of 0.5-5 μm, and there is a porous structure on the surface of nickel foam that is e...
Embodiment 2
[0034]Soak the nickel foam in a phytic acid solution with a pH of 8 regulated by potassium hydroxide, where the concentration of phytic acid is 5 mmol / L, and let it stand at room temperature for 2 hours. Then the nickel foam was transferred to the ferric sulfate solution and allowed to stand for 5 minutes, wherein the concentration of the ferric sulfate solution was 45mmol / L. Repeat the above process 2 times, take out the nickel foam, wash and dry. Finally, the modified nickel foam is directly used as an electrode, and the nickel-iron-based nanosheet / foam nickel oxygen evolution reaction electrode material is obtained by in-situ electrochemical activation by linear sweep voltammetry, and the scanning range is 1.0V-2.0V (vs. RHE), the sweep speed is 2mV / s, and the sweep is 10 times.
Embodiment 3
[0036] The pH of the phytic acid solution was adjusted to 3 by tris buffer solution, and the nickel foam was soaked in the above solution, wherein the concentration of phytic acid was 45mmol / L, and allowed to stand at room temperature for 5min. Then the nickel foam was transferred to ferric chloride solution and left to stand for 2h, wherein the concentration of ferric chloride solution was 5mmol / L. Repeat the above process 10 times, take out the nickel foam, wash and dry it. Finally, the modified nickel foam is directly used as an electrode, and the in-situ electrochemical activation is performed by a constant current method to obtain a nickel-iron-based nanosheet / nickel foam oxygen evolution reaction electrode material, where the current density is 500mA cm -2 , the time is 30min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com