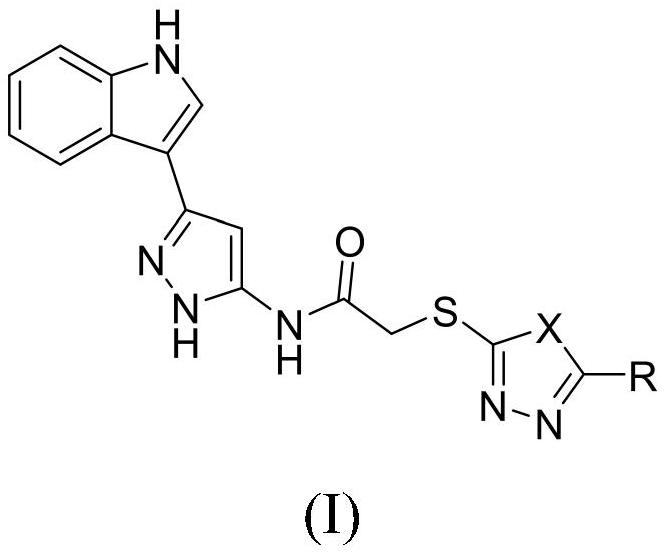
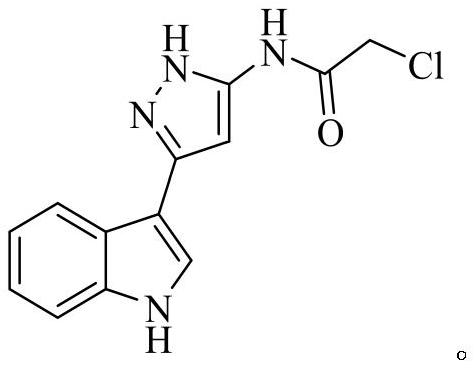
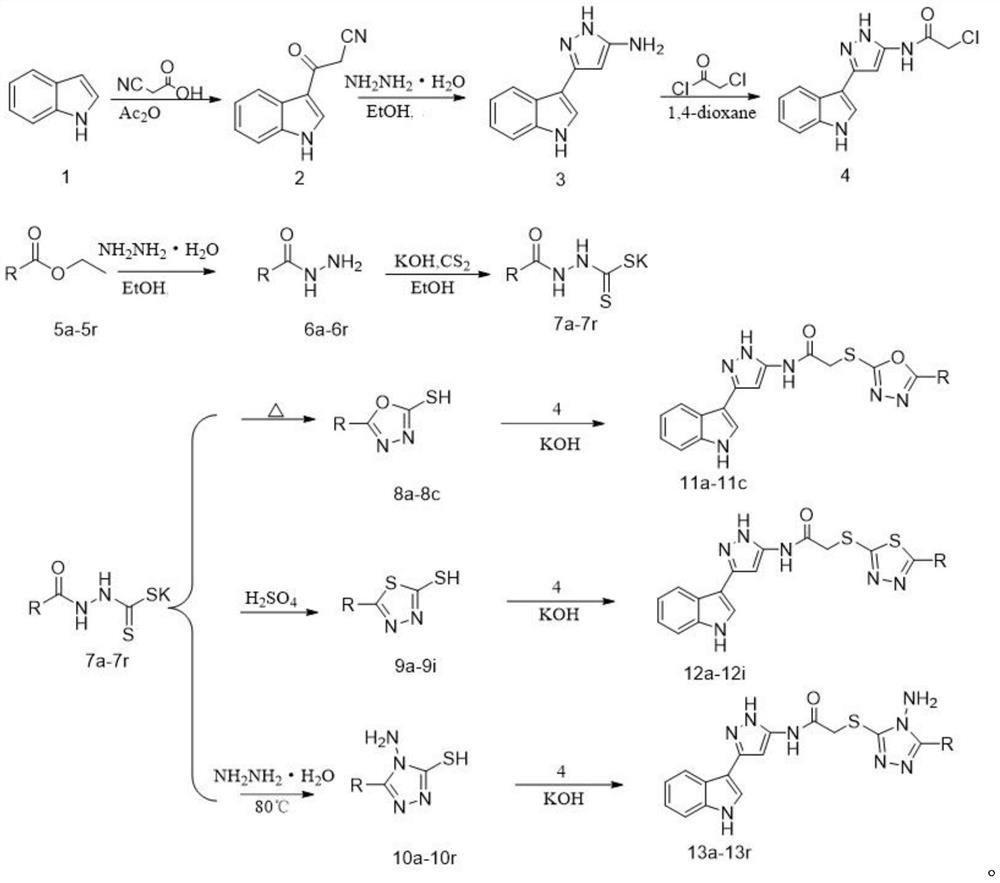
Indole derivative containing diazole, triazole and pyrazole structural units and application thereof
A technology of indole derivatives and structural units, applied in the field of indole derivatives, can solve the problem that the serious threat of cancer to human health has not been effectively controlled.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of 3-(1H-indol-3-yl)-3-oxo-propionitrile
[0046] Put indole (10.00g, 85.36mmol), cyanoacetic acid (10.17g, 119.50mmol) and 100mL acetic anhydride in a 250mL round bottom flask successively, and reflux at 40°C for 4h. After the reaction, it was cooled to room temperature, filtered with suction, washed with methanol for 2-3 times, and dried to obtain a white solid with a yield of 77.3%.
Embodiment 2
[0047] Example 2: Preparation of 3-(1H-indol-3-yl)-1H-pyrazol-5-amine
[0048] Add 3-(1H-indol-3-yl)-3-oxo-propionitrile (5.00g, 27.14mmol) and 80% hydrazine hydrate (6.79g, 169.38mmol) successively in a 500mL round bottom flask, Then add about 250 mL of absolute ethanol as a solvent, reflux and stir at 80°C, and monitor the reaction system by TLC. After the reaction was finished, let stand and cool naturally. The reaction mixture was concentrated under reduced pressure to a thick state, cooled, filtered with suction, and washed with ethyl acetate for 2-3 times to obtain a brown solid with a yield of 54.1%.
Embodiment 3
[0049] Example 3: Preparation of N-(3-(1H-indol-3-yl)-1H-pyrazol-5-yl)-2-chloroacetamide
[0050] Add 3 (2.00g, 10.09mmol) and anhydrous sodium carbonate (1.07g, 10.69mmol) respectively into a 50mL round bottom flask, add a part of 1,4-dioxane, and stir at room temperature. Then chloroacetyl chloride (1.14g, 10.09mmol) was dissolved in 2mL of 1,4-dioxane (20mL in total), and added dropwise into the round bottom flask, and the stirring was continued for 1h. After the reaction was monitored by TLC, take another beaker and add 100 mL of water, add NaCl, then pour crushed ice into the reaction mixture, stir until precipitation occurs, let stand, filter under reduced pressure, and wash with water to obtain a gray-green solid. After drying, recrystallization from ethanol afforded 4. White solid, 18% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com