Crude oil organic chlorine transfer agent and dechlorination method
A technology of organochlorine and transfer agent, applied in organic chemistry, chemical instruments and methods, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
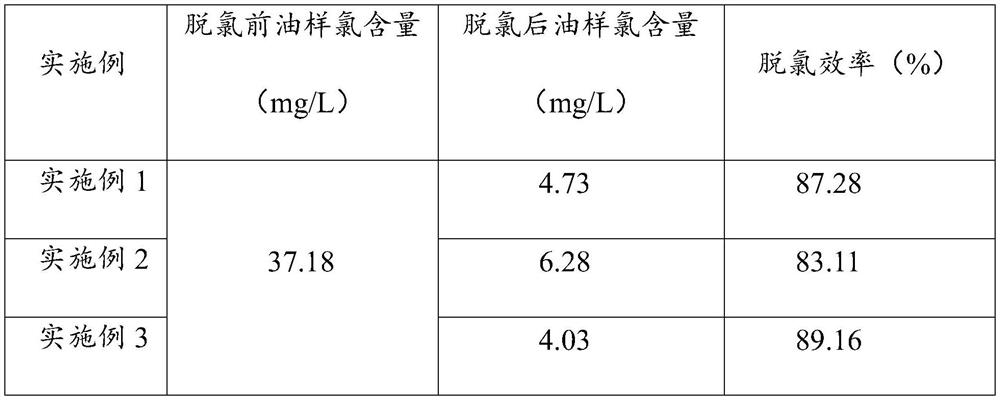
Embodiment 1
[0016] Preparation of organochlorine transfer agent: use absolute ethanol as the reaction solvent, the reactants are benzyltriethylammonium chloride and sodium hydroxide, add the solvent and the reactants to the three-necked flask in turn, install a thermometer, a stirring rod, and a condenser, Heating and heating, condensing and reflux, stirring and reacting, the reaction time is 2~5h, the reaction temperature is 60~90℃, the molar ratio of benzyltriethylammonium chloride and sodium hydroxide is 1:1~1:1.2, condense after the reaction to room temperature, and then use a sand core crucible to filter out sodium chloride to obtain benzyltriethylammonium hydroxide ethanol solution.
[0017] The chlorine content in the ethanol solution of benzyl ammonium hydroxide was detected to be 506 mg / L by microcoulometry.
[0018] Sodium hydroxide is selected as the nucleophile, and the molar ratio of crude oil organochlorine, organochlorine transfer agent, and nucleophile is 1:5:10.
[0019]...
Embodiment 2
[0021] Preparation of organochlorine transfer agent: using anhydrous methanol as the reaction solvent, the reactant is benzyltrimethylammonium chloride and potassium hydroxide, the solvent and the reactant are added to the three-necked flask in turn, and a thermometer, a stirring rod, and a condenser are installed. Heating and heating, condensing and reflux, stirring and reacting, the reaction time is 2 to 5 hours, the reaction temperature is 50 to 90°C, the molar ratio of benzyltrimethylammonium chloride to potassium hydroxide is 1:1 to 1:1.2, and condensed after the reaction to room temperature, and then filter potassium chloride with a sand core crucible to obtain benzyltrimethylammonium hydroxide methanol solution.
[0022] The chlorine content in the benzyltrimethylmethanol solution detected by microcoulometry was 470 mg / L.
[0023] Potassium hydroxide is selected as the nucleophile, and the molar ratio of crude oil organochlorine, organochlorine transfer agent, and nucle...
Embodiment 3
[0026] Preparation of organochlorine transfer agent: use absolute ethanol as the reaction solvent, the reactants are benzyltrimethylammonium chloride and sodium hydroxide, add the solvent and the reactants to the three-necked flask in turn, install a thermometer, a stirring rod, and a condenser, Heating and heating, condensing and reflux, stirring and reacting, the reaction time is 2 to 5 hours, the reaction temperature is 50 to 90°C, the molar ratio of benzyltrimethylammonium chloride and sodium hydroxide is 1:1 to 1:1.2, condense after the reaction to room temperature, and then use a sand core crucible to filter out sodium chloride to obtain benzyltrimethylammonium hydroxide ethanol solution.
[0027] The chlorine content in the ethanol solution of benzyltrimethylammonium hydroxide was detected to be 483mg / L by microcoulomb instrument.
[0028] Sodium ethoxide is selected as the nucleophile, and the molar ratio of crude oil organochlorine, organochlorine transfer agent, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com