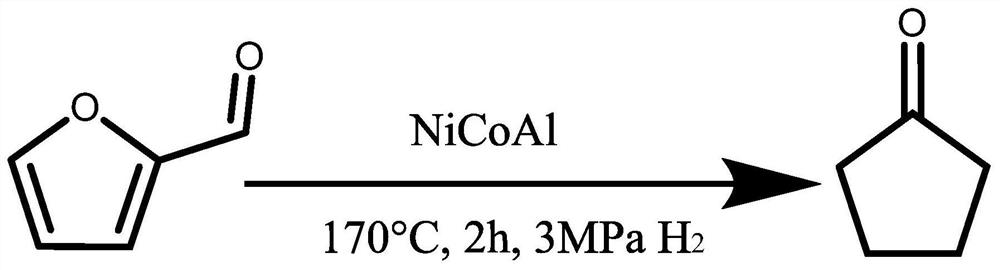
Method for preparing cyclopentanone and cyclopentanol through furfural hydrogenation rearrangement
A technology of furfural and preparation steps, which is applied in the field of preparation of cyclopentanol by catalytic hydrogenation and rearrangement of furfural, and the preparation of non-noble three-metal composite catalyst, which can solve the problems of high price, wide application, and lack of resources, and is beneficial to environmental protection , reduce production costs, broaden the effect of market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
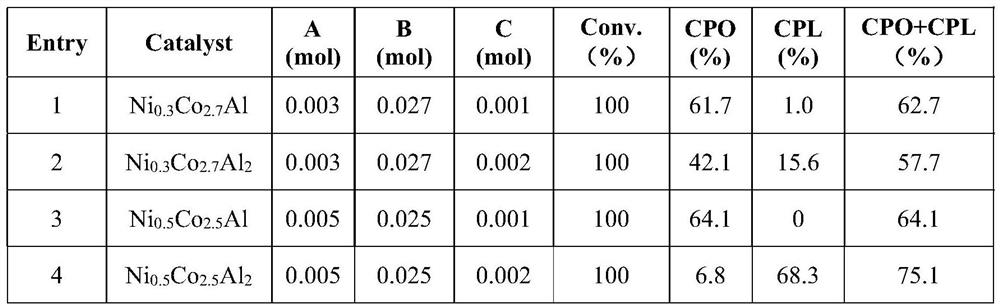
[0016] Catalyst preparation: Weigh 0.003mol of Ni (NO 3 ) 2 · 6h 2 O, 0.027mol of Co (NO 3 ) 2 · 6h 2 O and 0.001mol of Al 2 (NO 3 ) 3 · 9h 2 O was dissolved in 25mL of deionized water, is the solution A. Then excess urea was added 25mL of deionized water, i.e. solution B. The solution A and solution B was added to the heterogeneous reaction vessel was heated at 120 ℃ 4h, after the completion of the reaction, the reaction was taken gelatinous precipitate obtained was filtered and washed several times with deionized water until neutral precipitate . The precipitate was dried in an oven 12h, and then calcined at 500 deg.] C over 4h muffle furnace, reduction in the hydrogen atmosphere prior to use, to obtain three non-noble metal catalyst. The prepared catalyst was designated as NixCoyAlz, wherein x, y and z represent Ni, Co and Al atomic ratio.
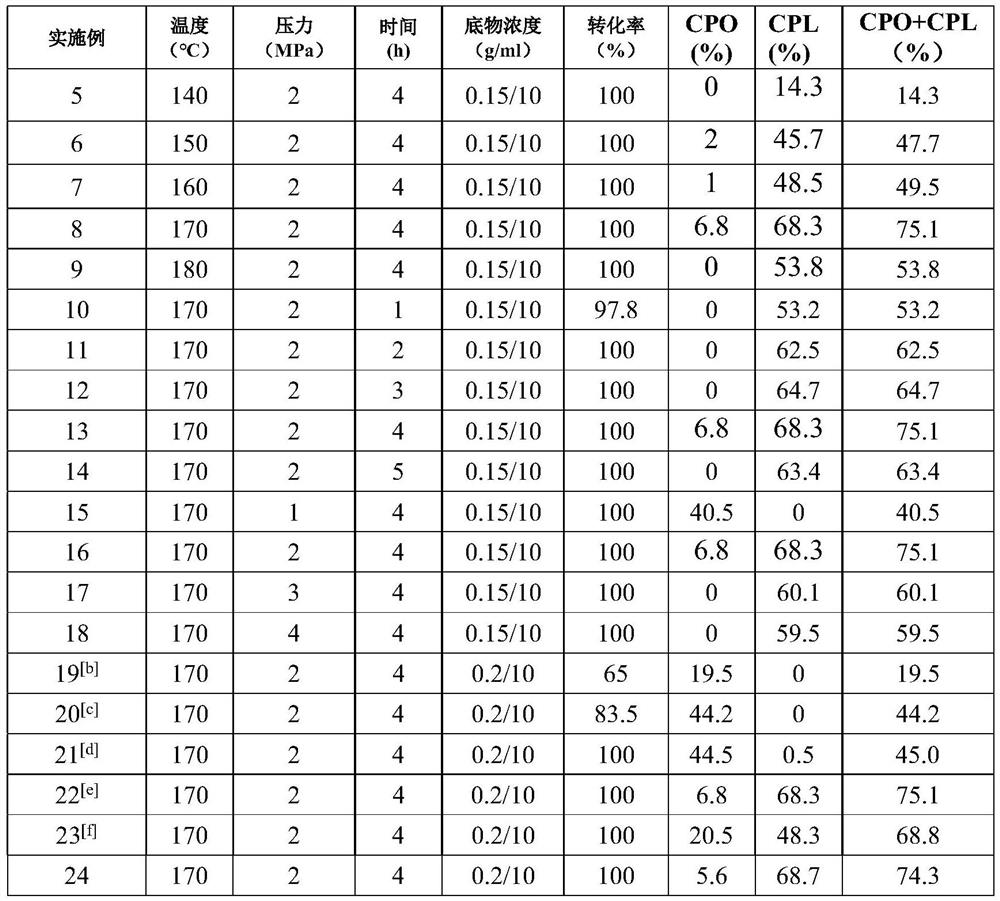
[0017] Cyclopentanol preparation: Weigh 0.15g furfural, 10mL of water and 0.06g of the catalyst (NixCoyAlz, where x = 0, y = 3, z = 1) and...
Embodiment 2
[0019] The method of preparing the same catalyst as in Example 1, but in which an Al 0.001mol 2 (NO 3 ) 3 · 9h 2 O molar amount changed to 0.002 mol. The catalyst and the reaction is recovered by a magnet through, for repeating cycle test.
[0020] The method of preparing the reference cyclopentanol in Example 1 were. The product by gas chromatography (7890B, Agilent) quantitative analysis by gas chromatography under the conditions in FIG as image 3 Indicated. 22-24min at peak represents cyclopentyl and cyclopentanol. As a result: furfural conversion was 100%, and the yield of cyclopentanone cyclopentanol 57.7%.
Embodiment 3-4
[0022] Respectively according to the method in Example 1 and Example 2 was prepared to obtain the corresponding three-metal of the metal catalyst, but the 0.003mol of Ni (NO 3 ) 2 · 6h 2 O, 0.027mol of Co (NO 3 ) 2 · 6h 2 O molar amounts were changed to 0.005mol and 0.025mol, namely Example 3 and Example 4.
[0023] The catalyst prepared above, was carried out as in Example 2, experiments determine the conversion rate and yield of the reaction product obtained, shown in Table 1.
[0024] Table 1 Effect of the preparation and rearrangement of furfural aqueous phase hydroprocessing cyclopentanol conversion rate and yield of different types of catalysts
[0025]
[0026] Wherein: A: Ni (NO 3 ) 2 · 6h 2 O; B: Co (NO 3 ) 2 · 6h 2 O; C: Al 2 (NO 3 ) 2 · 9h 2 O; Conv .: conversion; CPO: cyclopentanone; CPL: Cyclopentanol
[0027] Comprehensive Examples 1 to 4, the catalyst in NiCoAl, Ni 0.5 CO 2.5 Al exhibits the best activity, CPO and overall yield of up to 75.1% of the CPL. In partic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com