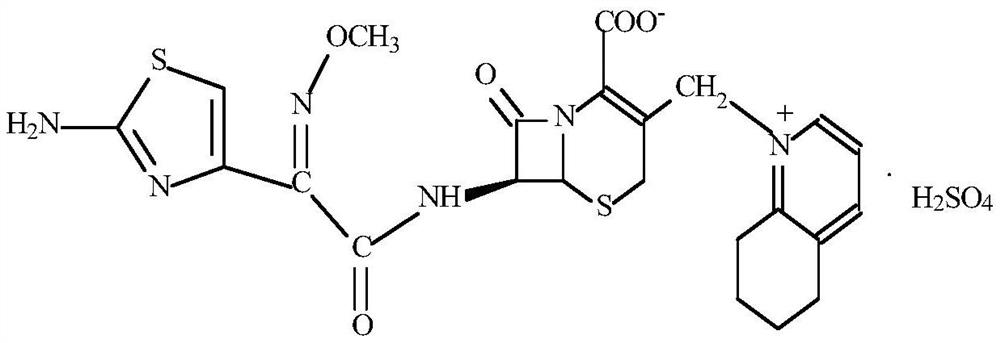
Preparation method of cefquinome sulfate intermediate 7-aminocefquinome
A technology of aminocefaquineme and cefquinoxime sulfate, which is applied in the field of preparation of cefquinoxime sulfate intermediate 7-aminocefaquineme, can solve the problems of slow material reaction, reduced solvent amount, low production efficiency, etc., and achieves reduction The effects of stirring the dead zone, increasing the reaction speed, and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
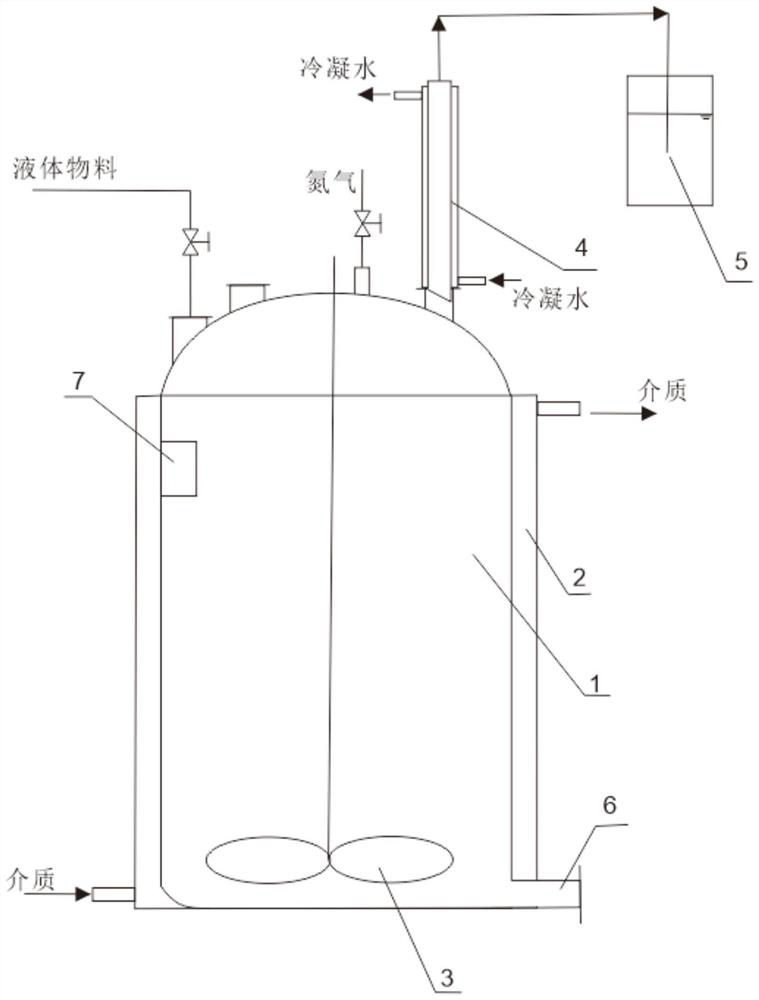
[0024] The alkylation reaction kettle includes a reaction kettle body 1, which is a flat-bottomed kettle, and a jacket 2 is provided outside, through which condensed water or a heating medium can be passed through to heat the inside of the kettle. There is a stirring 3 inside, and the stirring 3 extends to the bottom of the alkylation reaction kettle, and the top cover is provided with a feeding port (including a solid material feeding port and a liquid material feeding port, wherein a feeding tube is arranged on the liquid material feeding port), and the alkylation A condensation pipe 4 is inserted on the top cover of the chemical reaction kettle, and a jacket is provided outside the condensation pipe 4 for passing condensed water. The top outlet of the condensation pipe 4 is connected to an ammonia recovery pipe, and the ammonia gas recovery pipe is connected to the ammonia adsorption recovery device 5. Water is contained in the ammonia adsorption device 5 for absorbing the g...
Embodiment 2
[0029] Clean the alkylation reactor, dry it, and cool it for use.
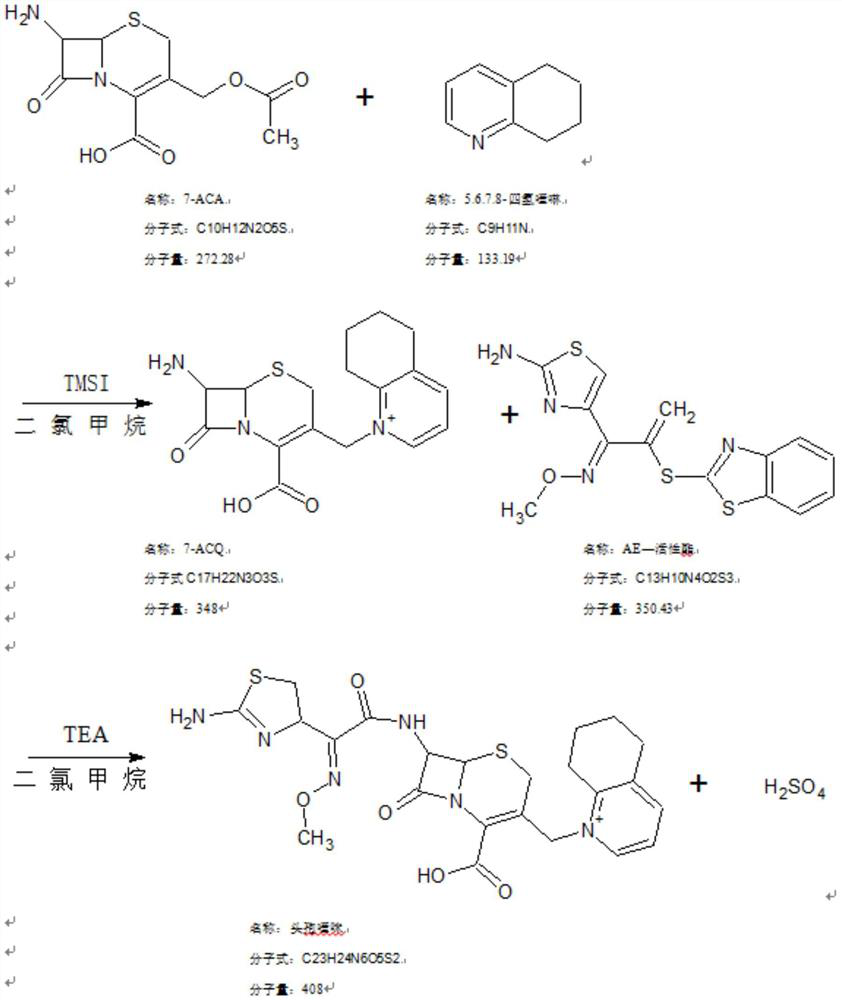
[0030] In a 500L alkylation reactor, add 85kg of dichloromethane, 19.5KG of hexamethyldisilazane, stir, add 25KG of 7-ACA plus 0.22KG of trimethylchlorosilane, stir, and raise the temperature to 53-57°C. The reaction was refluxed for 2 hours.
[0031] After the reaction is complete, lower the temperature to 3-7°C, add 25KG N,N-diethylaniline, and stir evenly. Add 28.5 kg of iodotrimethylsilane to the reaction kettle, and stir for 30 minutes. Steam was introduced to raise the temperature to 15-20°C, and the reaction was stirred for 2.5 hours. After the reaction is complete, lower the temperature to 3-7°C, add 4.5KG tetrahydrofuran, and stir for 20 minutes
[0032] 26KG 5,6,7,8-tetrahydroquinoline and 46.5KG N,N-dimethylacetamide were added, and the reaction was stirred for 6 hours.
[0033] After the reaction is complete, add 150KG dichloromethane, stir evenly, cool down to 3-7°C with chilled water, stir, o...
Embodiment 3
[0036] Clean the alkylation reactor, dry it, and cool it for use.
[0037] In a 500L alkylation reactor, add 85kg of dichloromethane, 19.5KG of hexamethyldisilazane, stir, add 25KG of 7-ACA plus 0.22KG of trimethylchlorosilane, stir, and raise the temperature to 53-57°C. The reaction was refluxed for 4 hours.
[0038] After the reaction is complete, lower the temperature to 3-7°C, add 25KG N,N-diethylaniline, and stir evenly. Add 28.5 kg of iodotrimethylsilane to the reaction kettle, and stir for 30 minutes. Steam was introduced to raise the temperature to 15-20°C, and the reaction was stirred for 2.5 hours. After the reaction is complete, lower the temperature to 3-7°C, add 4.5KG tetrahydrofuran, and stir for 20 minutes
[0039] 26KG 5,6,7,8-tetrahydroquinoline and 46.5KG N,N-dimethylacetamide were added, and the reaction was stirred for 6 hours.
[0040] After the reaction is complete, add 150KG dichloromethane, stir evenly, pour chilled water to cool down to 3-7°C, stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com