Method for continuously producing 6-chloro-2, 4-dinitroaniline and diazonium salt thereof
A technology of dinitroaniline and diazotization, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of cumbersome operation steps and low production efficiency, so as to simplify the process flow and improve production efficiency , to achieve the effect of economic and environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] (continuous production of 6-chloro-2,4-dinitroaniline)
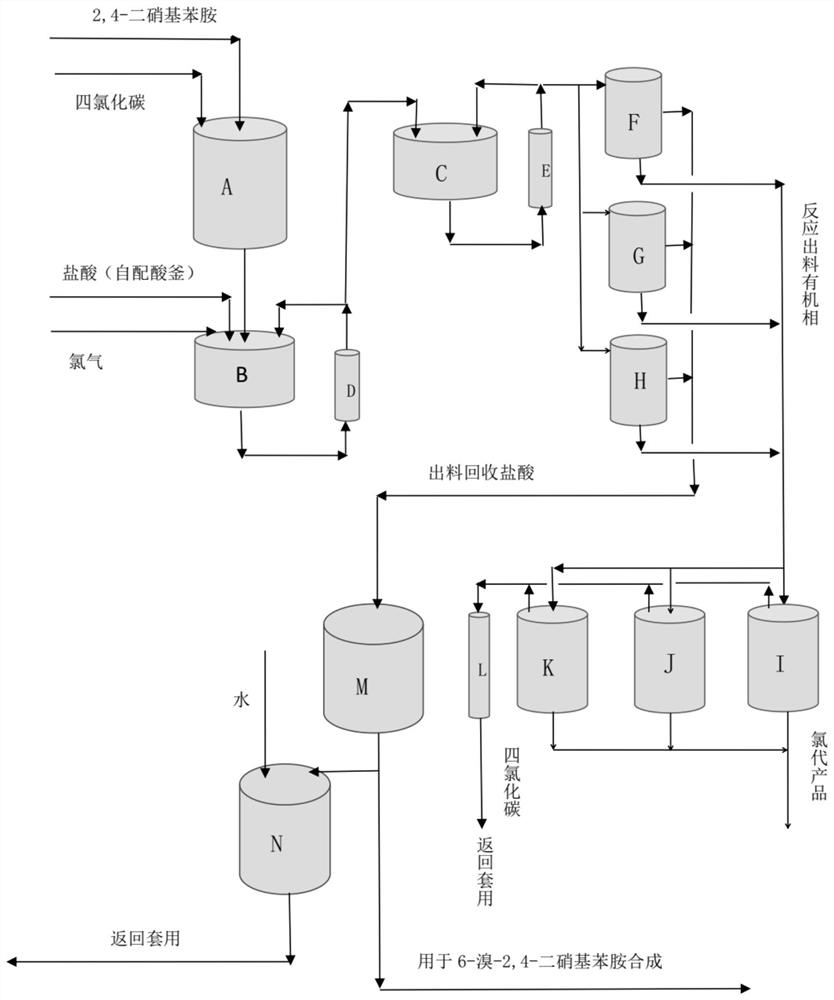
[0080] Such as figure 1 As shown, two series of high-gravity reactors B and C, three parallel stratified tanks F, G and H, and three parallel distillation tanks I, J and K are used. Among them, the volumes of the supergravity reactors B and C are both 500L, the chlorination reaction temperature is 40°C-45°C, the volumes of the layered tanks F, G, and H are all 1000L, and the volumes of the distillation tanks I, J, and K are all 1000L. is 1500L, the volume of hydrochloric acid recovery tank M is 1500L, the volume of condenser L is 500L, the volume of acid mixing tank N is 1000L, and the liquid level setting value of stratified tanks F, G and H is 90% of its volume. The liquid level setting value of distillation tank I, J and K is 50% of its volume.
[0081] In batching kettle A (volume 1500L), add 2,4-dinitroaniline and carbon tetrachloride continuously simultaneously, and the feeding speed of the two is respecti...
Embodiment 2
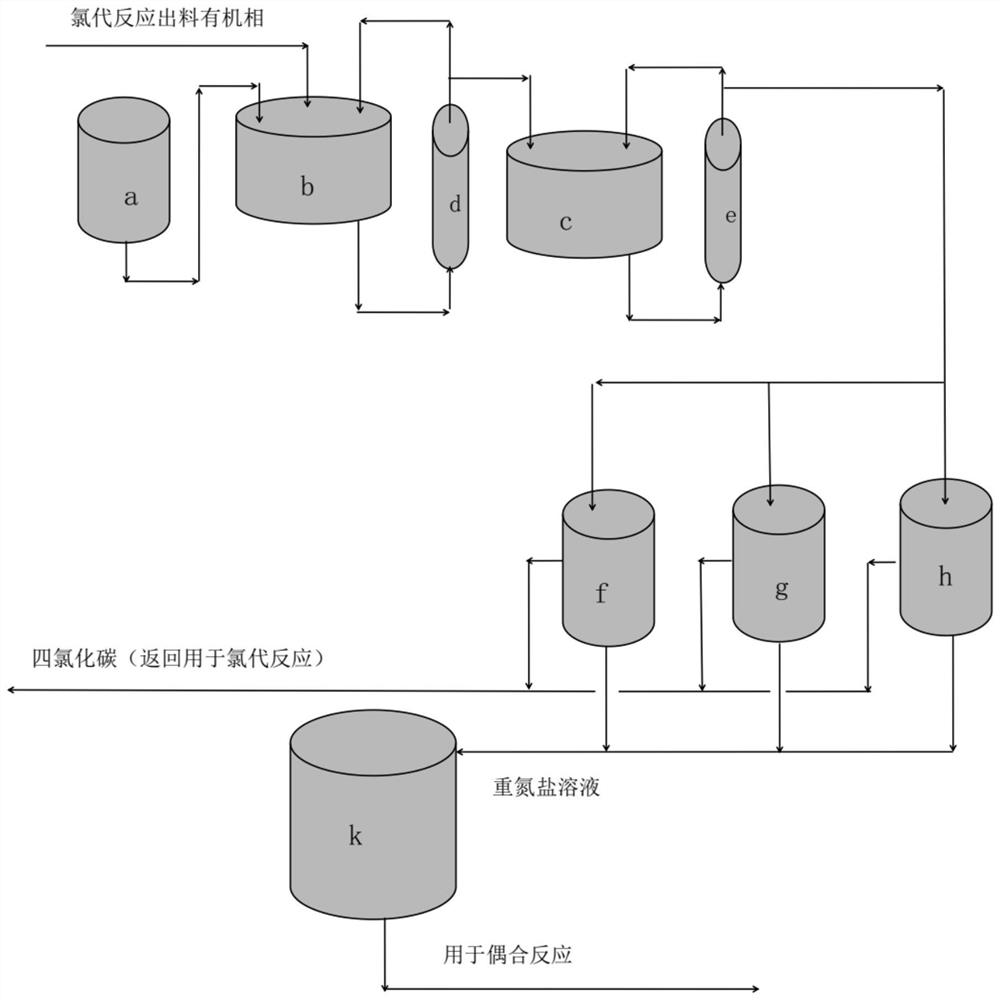
[0089] Repeat the method of Example 1 to obtain an organic phase comprising 6-chloro-2,4-dinitroaniline, followed by figure 2 As shown, the organic phase is not distilled, but is directly injected into the two-stage series high-gravity reactors b and c together with nitrosyl sulfuric acid with a mass concentration of 25% from the nitrosyl sulfuric acid storage tank a to carry out the diazotization reaction . Among them, the feed rates of the organic phase and nitrosyl sulfuric acid are 566.2kg / h and 254.2kg / h respectively, the volumes of the high-gravity reactors b and c are both 25L, and the diazotization reaction temperature is 18°C-25°C , the volume of stratified tanks h, g and f is 100L, the volume of diazonium salt solution storage tank k is 300L, and the liquid level setting value of stratified tanks h, g and f is 80% of its volume.
[0090] Wherein, a part of the material in the primary supergravity reactor b passes through the circulation pump (not shown) and then re...
Embodiment 3
[0094] (continuous production of 6-chloro-2,4-dinitroaniline)
[0095] Repeat the method of Example 1, except for the following steps, and the rest of the steps are the same as in Example 1.
[0096] Add 2,4-dinitroaniline and carbon tetrachloride continuously in batching kettle A (volume 500L) at the same time, and the feeding speeds of the two are respectively 110.02kg / h and 550.10kg / h; The carbon tetrachloride solution of nitroaniline is injected into the primary supergravity reactor B at a speed of 660.12kg / h. Simultaneously, mass concentration is that the hydrochloric acid of 16% is 547.5kg / h with feed rate, and chlorine is passed into the primary supergravity reactor B with the flow rate of 42.6kg / h. A part of the material in the primary supergravity reactor B passes through the circulating pump (not shown) and returns to the primary supergravity reactor B through the heat exchanger D, and the other part enters the secondary stage at a flow rate of 1250.22kg / h. Hypergr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com