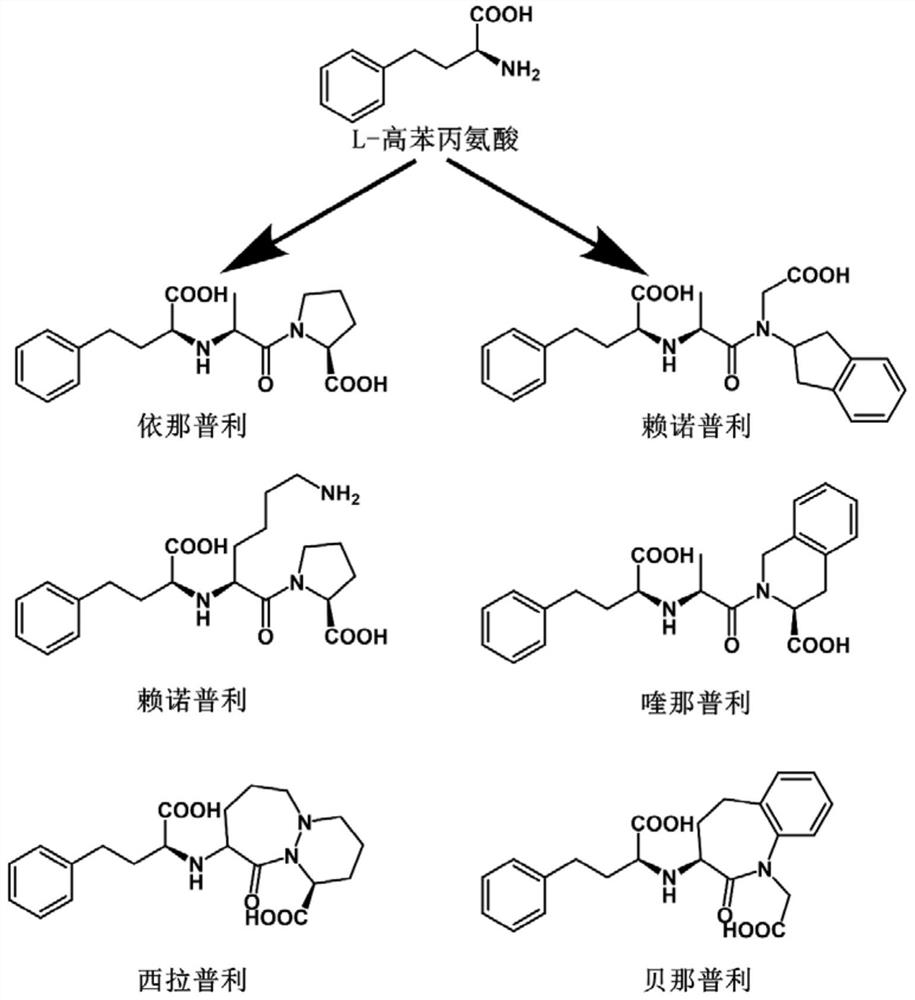
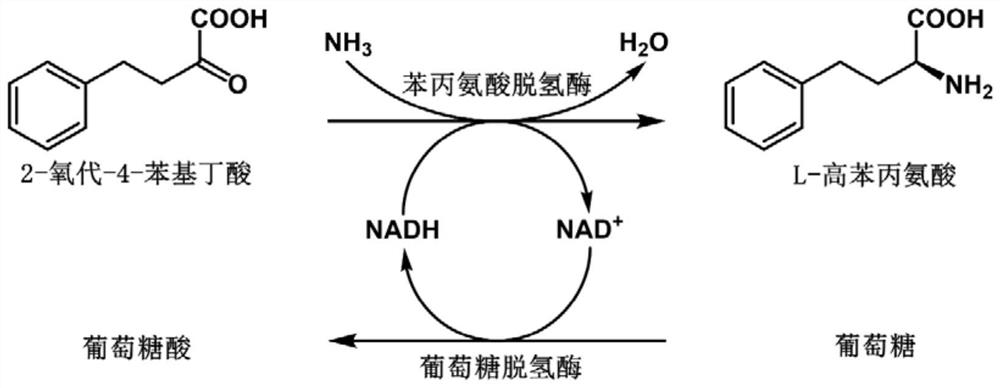
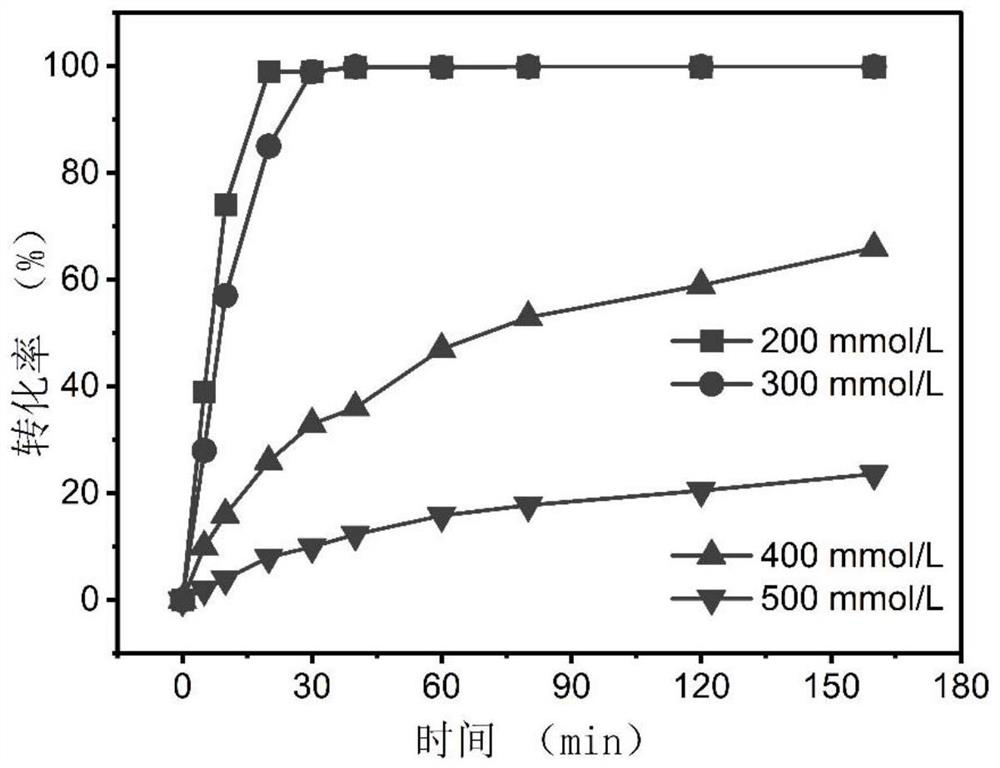
Phenylalanine dehydrogenase mutant and application thereof in synthesis of L-homophenylalanine
A technology of phenylalanine dehydrogenase and high phenylalanine, which is applied in the field of enzyme engineering and microbial engineering to achieve good temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of Phenylalanine Dehydrogenase mutant gene sequence
[0052] Specific steps are as follows:
[0053] Refer to the literature "Asano Y, Nakazawa A, Endo K, et al.Phenylana manufacturer Dehydrogenaseof Bacillus Badius.purification, Characterization and Gene Cloning [J] .EUR JBIOCHEM, 1987, 168 (1): 153-159." Chemical synthetic amino acid sequence such as The wild-type phenylalanine dehydrogenase shown in SEQ ID NO.1, the nucleotide sequence encoding the wild-type phenylanine dehydrogenase gene is shown in SEQ ID NO.2.
[0054] The obtained gene encoding the wild-type phenylalanine dehydrogenase was ligated by both the PET-28A (+) plasmid after bisase digestion (XHO I, BamHi), and the recombinant plasmid PET28A-PHEDH was obtained, and E. coli E was transformed. Coli BL21 (DE3) obtained recombinant E. coli PET28A-PHEDH / E.COLI BL21.
[0055] Using the total plasmid PCR technology, the obtained recombinant plasmid PET28A-PHEDH was used as a template to ob...
Embodiment 2
[0073] Example 2: Inducing culture and protein purification of phenylalanine dehydrogenase mutants
[0074] The recombinant E. coli PET28A-PHEDH / E.Coli BL21 obtained in Example 1 and a gene encoding mutant L306V, V309A, V309G, V309G / L50V, V309G / L306V, V309G / L306V / V144A, and V309G / L306V / V144G. The recombinant E. coli was applied to the LB solid medium, cultured from 8 to 10 h at 37 ° C to obtain a single colony; picking a single colony into an LB liquid medium, from 37 ° C, 200 rpm culture 6 to 8 h, obtained seed fluid; After 2% (V / V) of the inoculation amount, the LB liquid medium was added to 37 ° C, 200 rpm in 2 to 3 h hours, IPTG of the final concentration of 0.1 mmol / L, 2 ° C, 200 rpm continued to induce the culture 12 To 17h, a fermentationery was obtained; the fermentationery solution was centrifuged at 4 ° C, 8000 rpm for 5 minutes, discarded, washed with 9% saline, washed twice, and obtained wild type, mutant L306V, V309A, V309G, V309G / L50V, V309 g / l3...
Embodiment 3
[0076] Example 3: Catalytic efficiency of different phenylalanine dehydrogenase mutants on 2-oxo-4-phenylbousic acid
[0077] Specific steps are as follows:
[0078] Different benzenes were obtained at different concentrations (0.1 to 25 mmol / L2-oxo-4-phenylbutyric acid). Catalytic efficiency of alanine dehydrogenase.
[0079] In NH 4 CL-NH 4 2-oxo-4-phenylbucic acid (0.1 to 25 mmol / L) and NADH (0.5 mmol / L) were added to the OH buffer (1 mol / L, pH 9.0), and the reaction system was obtained; the reaction system was constructed in 30 After 2 min at ° C, 20 μl of the wild type, mutant, V309 g / L306V / V144A, and V309G / L306V / V1444, and V309 g / L306V / V144 g, and V309 g / L306V / V144 g, respectively. The concentrated enzyme solution is not contained, the other components are the same; 50 ° C reaction 5 min, each 10s recording the absorbance of the reaction system at 340 nm at 340 nm, and calculates the wild type, mutant L306V, V309A, V309G, V309G / L50V, V309G / The cat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com