Method for full-liquid-phase synthesis of deslorelin
A liquid-phase synthesis and deslorelin technology, applied in the field of medicine, can solve the problems of low crude product purity, high environmental protection pressure, pressure on large-scale production costs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
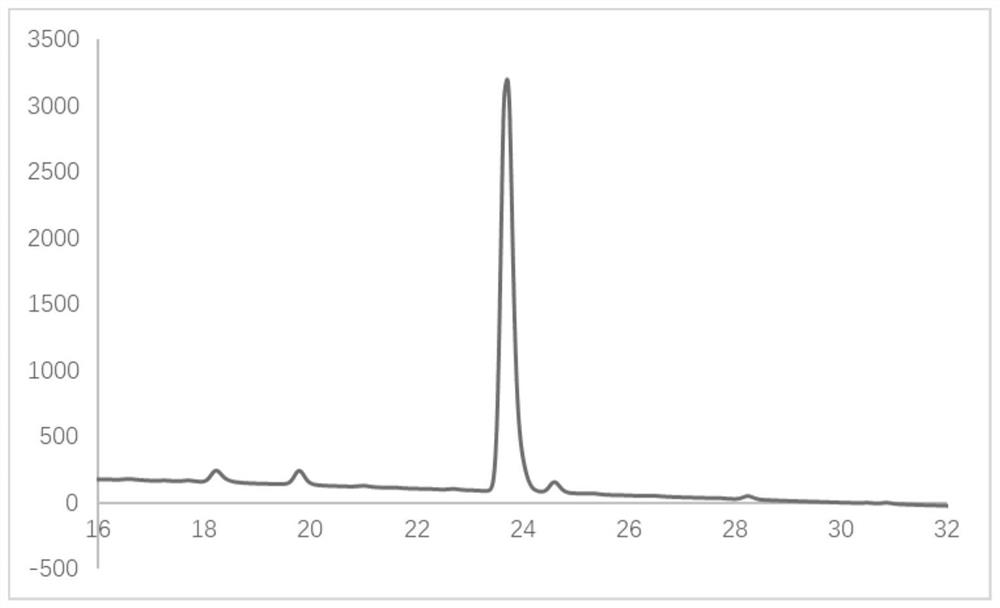
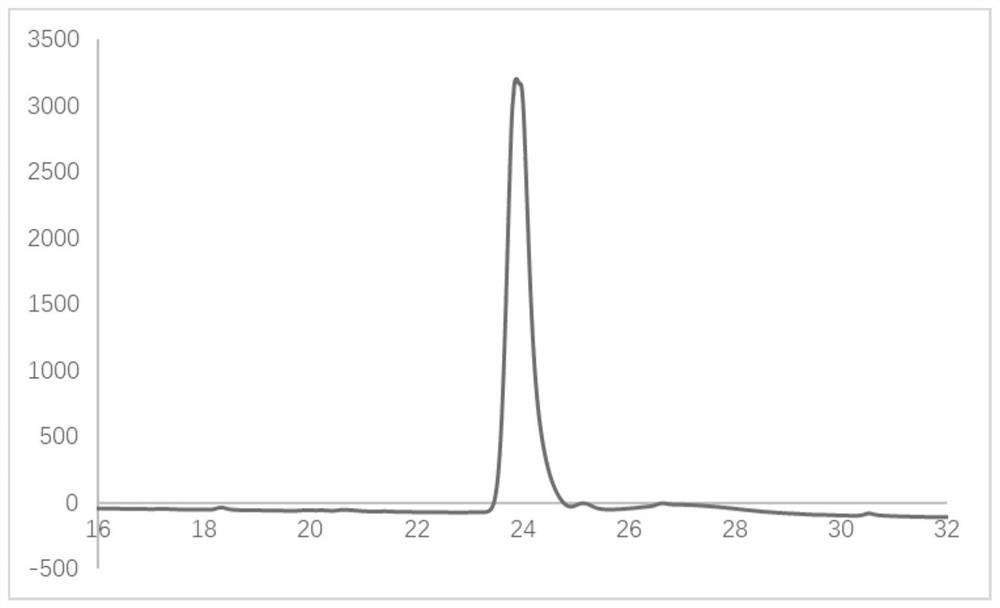
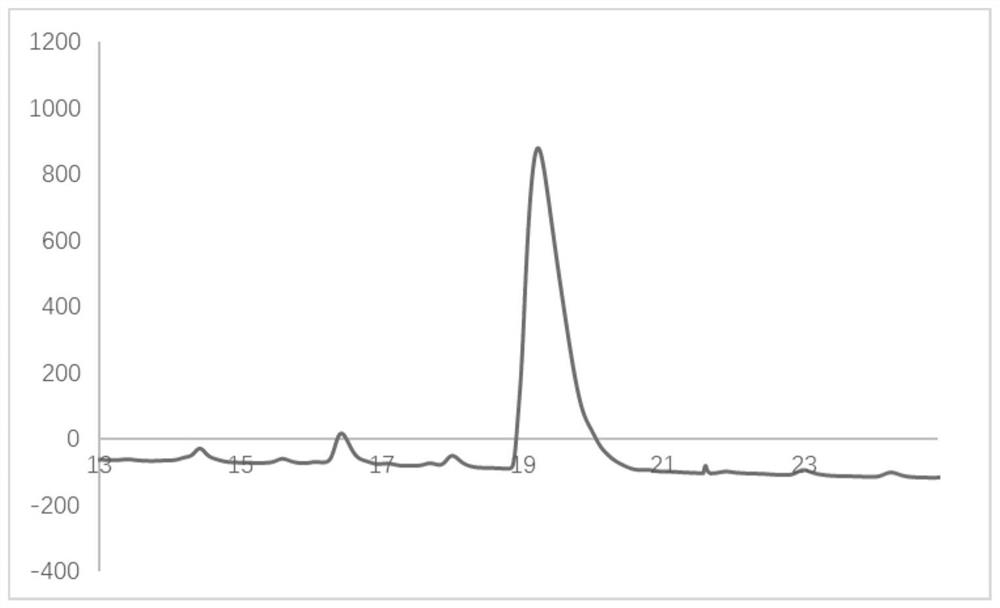
Image
Examples
Embodiment 1
[0062] 1. Synthesis of compound 1: Fmoc-Trp(Boc)-Ser(tBu)-Tyr(tBu)-OH
[0063] 1.1 Feeding:
[0064] Feed according to the materials in Table 2.
[0065] Table 2 Types and dosage of materials
[0066] abbreviation Dosage Fmoc-Trp(Boc)-Ser(tBu)-OSU 100mmol H-Tyr(tBu)-OH 110mmol TEA 110mmol DMF 400ml 0.5M hydrochloric acid solution 1L
[0067] 1.2 Operation process
[0068] After the synthesized Fmoc-Trp(Boc)-Ser(tBu)-OSu was completely dissolved in DMF, the H-Tyr(tBu)-OH was accurately weighed and added to the above reaction flask, and TEA 110mmol was added to start the reaction. After stirring and reacting for 60 min, HPLC detected that the reaction was complete.
[0069] The reaction solution was poured into the Erlenmeyer flask twice, and then 0.5M hydrochloric acid was added to rapidly stir and precipitate, and the filtered solid was then washed with purified water until neutral, and dried at 30°C. Collect the solids int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com