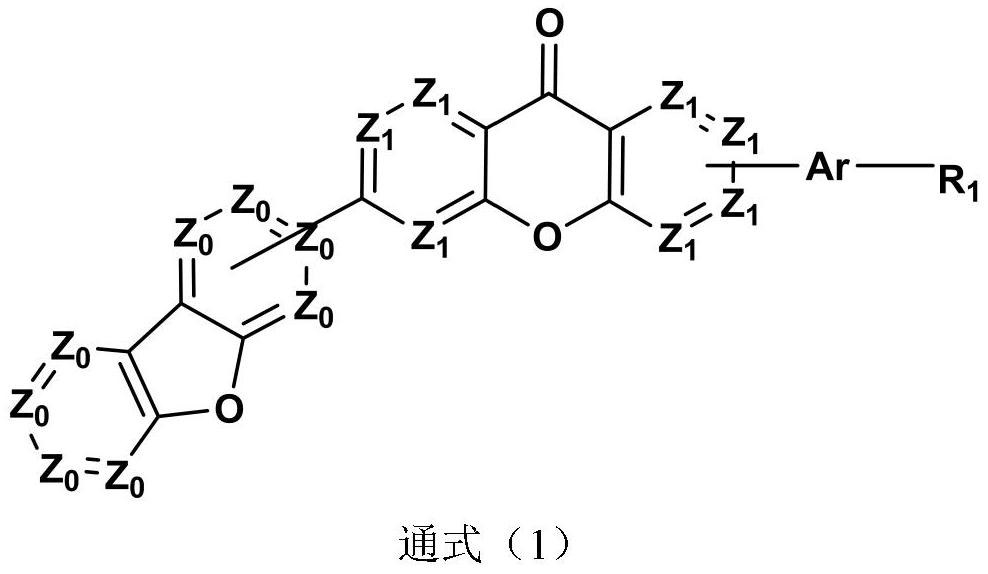
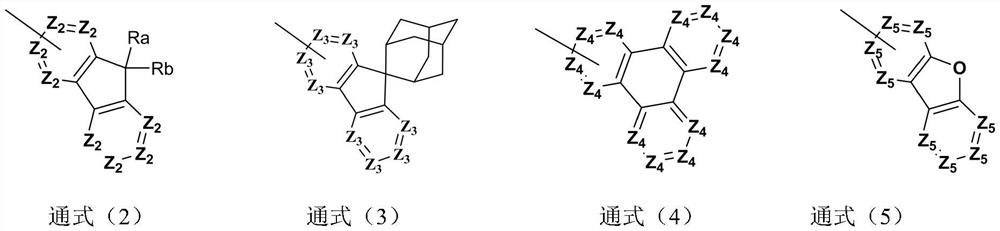
Compound taking xanthone-linked dibenzofuran as core and application thereof
A technology of benzofuran and xanthone, which is applied in the fields of organic chemistry, chemical instruments and methods, electrical components, etc., can solve the problems of different performances, etc., and achieves improved processing window, improved stability, and strong stereo rigidity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
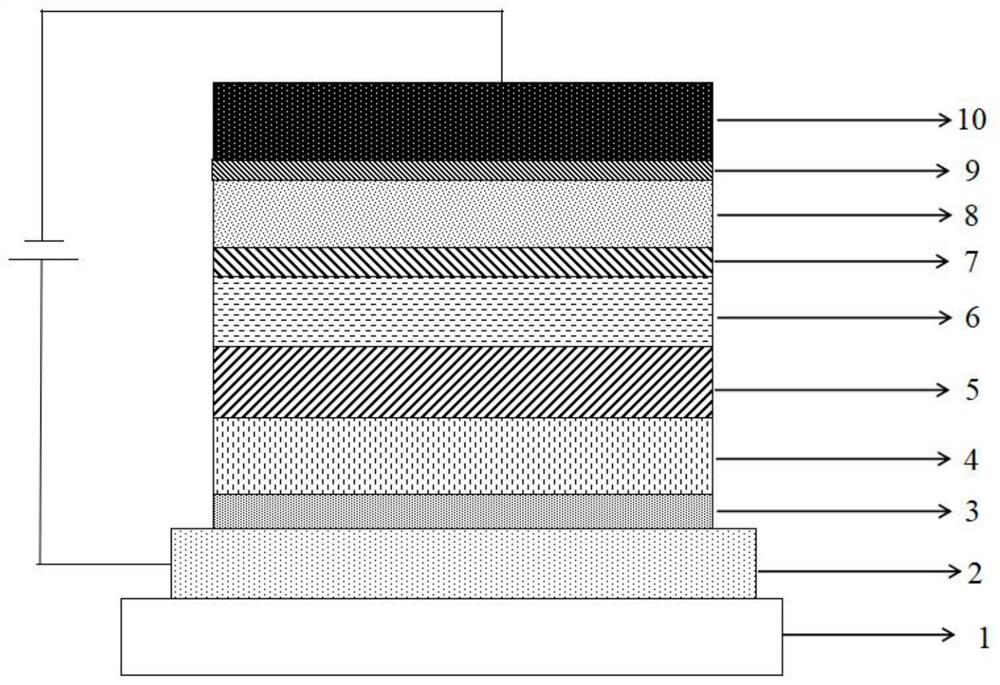
Image
Examples
Embodiment 1
[0059] The synthesis of embodiment 1 compound 3:
[0060]
[0061] Add 0.01mol of raw material 1-1 and 0.012mol of raw material 2-1 to 120mL of toluene:ethanol=2:1 mixed solvent, add 0.02mol of potassium carbonate, add 0.0002mol of Pd(PPh 3 ) 4 , reacted at 110°C for 48 hours under a nitrogen atmosphere, took a sample and spotted the plate, after the reactants were completely reacted, cooled and filtered, the filtrate was rotary evaporated to remove the solvent, and the crude product was passed through a silica gel column to obtain compound 3; HPLC purity 98.41%, yield The rate is 77.52%. Elemental analysis structure (molecular formula C 49 h 28 o 4 ): theoretical value: C, 86.45; H, 4.15; test value C, 86.48; H, 4.12. MS (m / z): Calculated 680.20, found 680.39. 1 HNMR (CDCl 3 ,400MHz)δ7.91(d,2H),7.72(d,2H),7.66(d,2H),7.62–7.56(m,4H),7.50–7.34(m,16H),7.07(d,2H) .
Embodiment 2
[0062] The synthesis of embodiment 2 compound 20:
[0063]
[0064] The preparation method of compound 20 was the same as that in Example 1, except that the reactant 2-1 was replaced with the starting material 2-2 to obtain compound 20; the HPLC purity was 98.89%, and the yield was 70.61%. Elemental analysis structure (molecular formula C 45 h 24 o 4 ): theoretical value: C, 85.97; H, 3.85; test value C, 85.96; H, 3.86. MS (m / z): Calculated 628.17, found 628.44. 1 H NMR (CDCl 3 ,400MHz)δ8.04(ddd,4H),7.95(d,2H),7.80(dt,2H),7.61–7.55(m,4H),7.52–7.32(m,10H),7.07(d,2H) .
Embodiment 3
[0065] The synthesis of embodiment 3 compound 137:
[0066]
[0067] The preparation method of compound 137 was the same as that in Example 1, except that the reactant 2-1 was replaced with the starting material 2-5 to obtain compound 3; the HPLC purity was 97.52%, and the yield was 78.36%. Elemental analysis structure (molecular formula C 45 h 24 N 4 o 4 ): theoretical value: C, 78.94; H, 3.53; N, 8.18; test value C, 78.94; H, 3.53; N, 8.18. MS (m / z): Calculated 684.18, found 684.31. 1 H NMR (CDCl 3 ,400MHz)δ8.91(s,2H),7.96(d,2H),7.60–7.52(m,4H),7.51(dd,2H),7.47–7.34(m,12H),7.07(d,2H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com