Captopril sustained-release microcapsule and preparation method thereof
A technology of sustained-release microcapsules and inner core layers, applied in microcapsules, capsule delivery, pharmaceutical formulations, etc., can solve problems such as unfavorable human health, dizziness, headaches, gastrointestinal disorders, etc., to improve sustained-release performance and prolong storage. Duration, the effect of improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
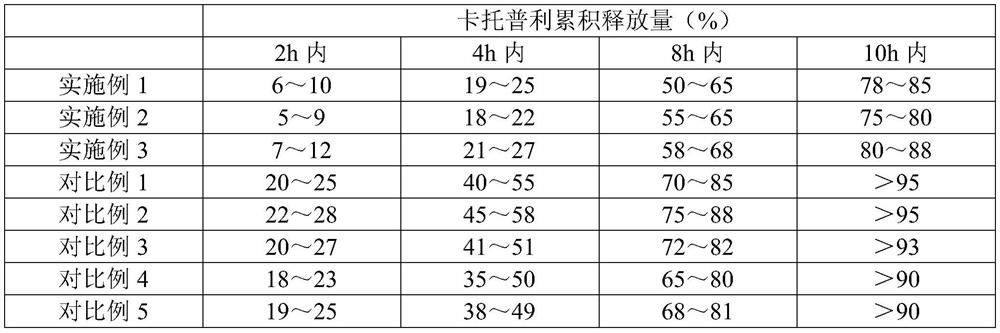
Embodiment 1
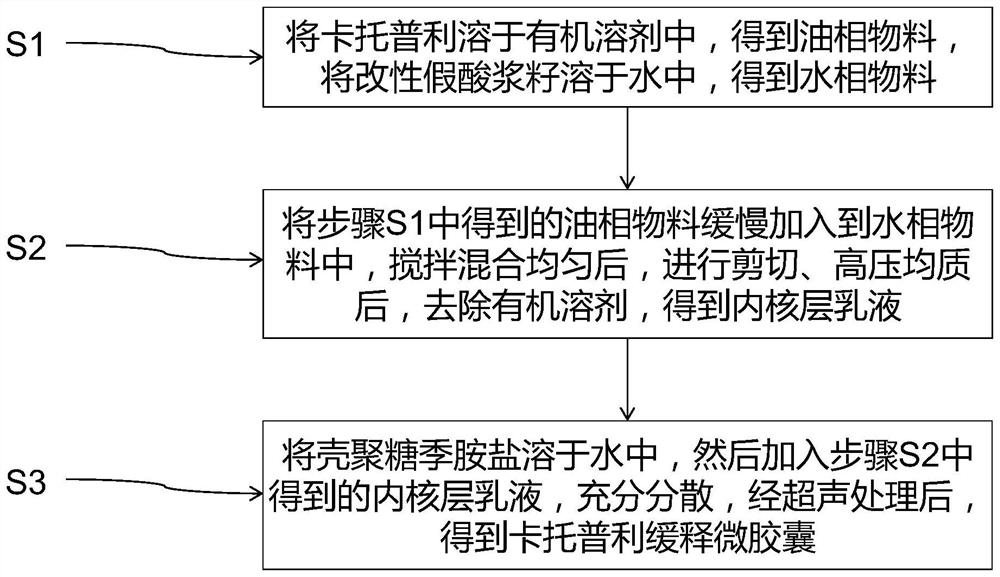
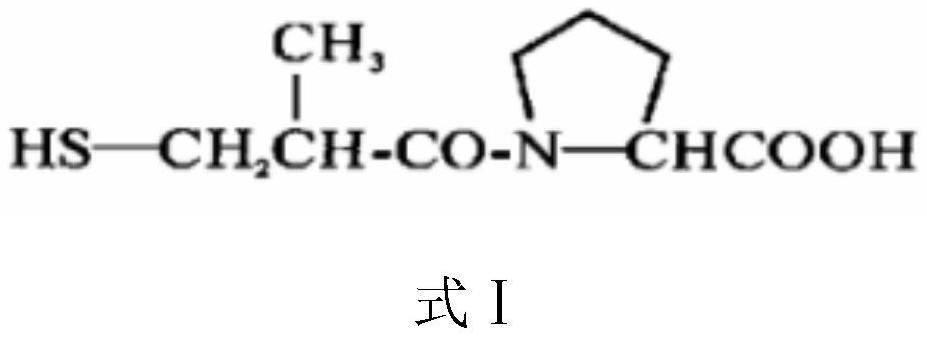
[0038] A captopril slow-release microcapsules, comprising an outer shell and an inner core layer, the inner core layer is a modified pseudophysalis seed material, the inner core layer wraps captopril, and the outer shell layer is chitosan quaternary An amine salt material, the outer shell layer covers the inner core layer. The mass ratio of the modified pseudophysalis seeds, the captopril and the chitosan quaternary ammonium salt is 1:0.5:1.5.
[0039] The modified pseudophysalis seeds are prepared through chemical modification and graft modification.
[0040] The specific operation method of the chemical modification is as follows: dry, pulverize and sieve the pseudophysalis seeds, add an aqueous solution 20 to 30 times the weight of the pseudophysalis seed powder and swell for 36 to 60 hours, and then add an acidic pH regulator Adjust the pH to 3-6, hydrolyze at 90-100° C. for 10-24 hours, and finally concentrate under vacuum to 15-30% of the original volume to obtain a con...
Embodiment 2
[0047] A captopril slow-release microcapsules, comprising an outer shell and an inner core layer, the inner core layer is a modified pseudophysalis seed material, the inner core layer wraps captopril, and the outer shell layer is chitosan quaternary An amine salt material, the outer shell layer covers the inner core layer. The mass ratio of the modified pseudophysalis seeds, the captopril and the chitosan quaternary ammonium salt is 1.5:0.7:2.5.
[0048] The modified pseudophysalis seeds are prepared through chemical modification and graft modification.
[0049] The specific operation method of the chemical modification is as follows: dry, pulverize and sieve the pseudophysalis seeds, add an aqueous solution 20 to 30 times the weight of the pseudophysalis seed powder and swell for 36 to 60 hours, and then add alkaline pH adjustment The pH is adjusted to 8-10 with an agent, hydrolyzed at a temperature of 90-100°C for 10-24 hours, and finally concentrated under vacuum to 15-30%...
Embodiment 3
[0056] A captopril slow-release microcapsules, comprising an outer shell and an inner core layer, the inner core layer is a modified pseudophysalis seed material, the inner core layer wraps captopril, and the outer shell layer is chitosan quaternary An amine salt material, the outer shell layer covers the inner core layer. The mass ratio of the modified pseudophysalis seeds, the captopril and the chitosan quaternary ammonium salt is 2:0.8:3.
[0057] The modified pseudophysalis seeds are prepared through chemical modification and graft modification.
[0058] The specific operation method of the chemical modification is as follows: dry, pulverize and sieve the pseudophysalis seeds, add an aqueous solution 20 to 30 times the weight of the pseudophysalis seed powder and swell for 36 to 60 hours, and then add an acidic pH regulator Adjust the pH to 3-6, hydrolyze at 90-100° C. for 10-24 hours, and finally concentrate under vacuum to 15-30% of the original volume to obtain a conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com