Sulfur anion doped manganese dioxide material, preparation and application thereof, and zinc ion battery containing sulfur anion doped manganese dioxide material
A technology of zinc ion battery and manganese dioxide, which is applied in the field of electrochemical energy storage, can solve the problems of lithium ore resource shortage, price rise, flammability and safety, etc., and achieve improved zinc storage capacity, improved conductivity, and excellent energy storage performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] In this example, a sulfur anion-doped manganese dioxide material is prepared by the following method:
[0051] Step A: Weigh 0.6g (4mmol) KMnO 4 Dissolve 4mmol / L KMnO in 80mL deionized water 4 solution;
[0052] Step B: Configure the KMnO configured in step A 4 Move the solution into a 100mL autoclave, seal it, put it in an oven at 180°C, take it out after 2 hours of reaction, wash the reaction product 3 times with deionized water, and wash it once with absolute ethanol, put the precipitate in an oven at 60°C to dry 12h, the manganese dioxide precursor was obtained.
[0053] Step C: 13 mg (0.37 mmol) of sublimed sulfur powder was used as the sulfur source and 30 mg of the powdered MnO prepared in Step B 2 (4mmol) The precursor is placed in a quartz tube protected by an inert gas for sulfuration reaction, wherein the sublimed sulfur is placed at the gas inlet end of the quartz tube, and the MnO 2 The powder was placed at the gas outlet, and the quartz tube was heate...
Embodiment 2
[0079] In Example 2, a sulfur anion-doped manganese dioxide material was prepared by the following method: the method in Example 1 was adopted, and the only difference was that other condition parameters were changed to those shown in Table 2 below.
[0080] Table 2
[0081]
[0082] The sulfide anion-doped manganese dioxide material prepared in the above-mentioned embodiment 2 is characterized:
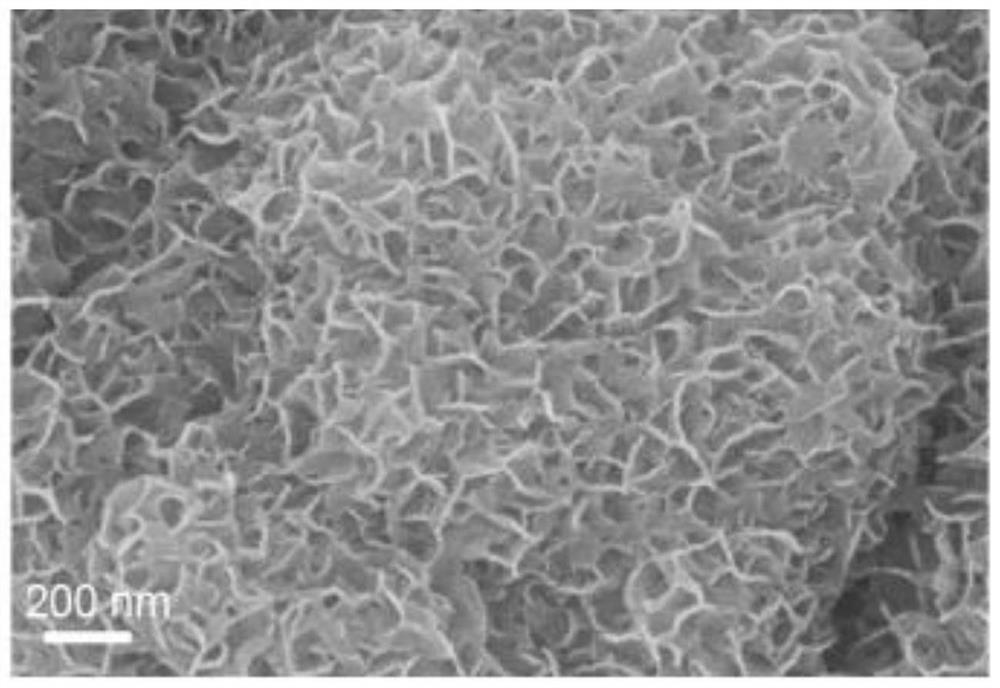
[0083] Figure 9 Scanning electron micrograph (SEM) of the sulfur anion-doped manganese dioxide electrode material prepared from manganese dioxide as a precursor obtained in Example 2 of the invention.
[0084] Figure 10X-ray powder diffraction pattern (XRD) under the preparation conditions for Example 2 of the present invention. The abscissa is 2θ, unit: degree; the ordinate is intensity. The curves are manganese dioxide and sulfur ion doped manganese dioxide powder.
[0085] From SEM Figure 9 It can be seen that the MnO 2 The flake structure is maintained before and aft...
Embodiment 3
[0093] In Example 3, a sulfur anion-doped manganese dioxide material was prepared by the following method:
[0094] Using the method of Example 1, the only difference is that other condition parameters are changed as shown in Table 4 below.
[0095] Table 4
[0096]
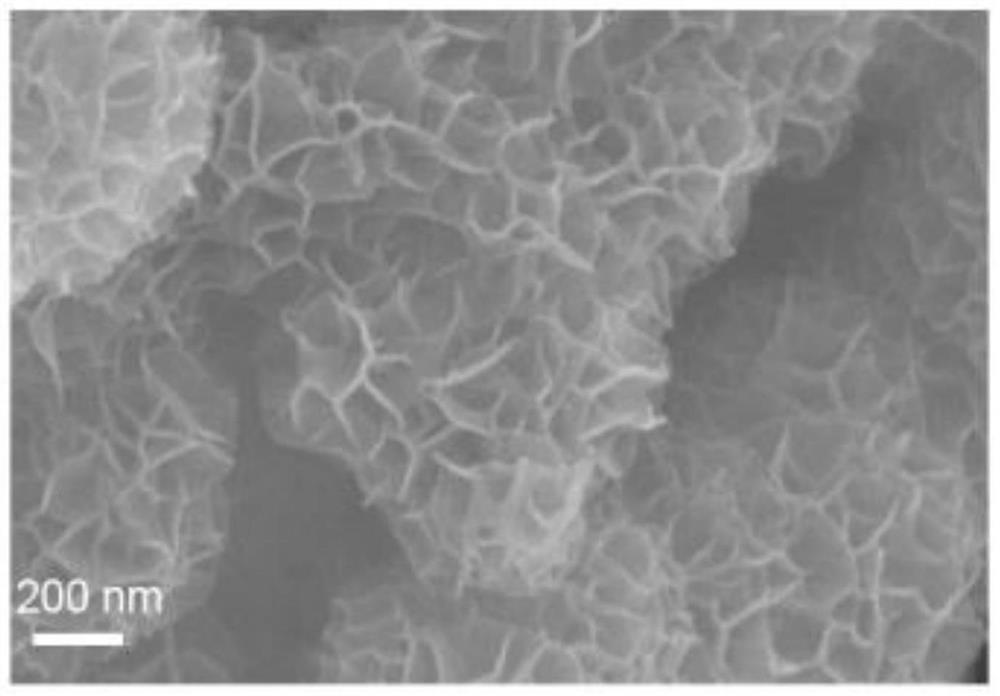
[0097] Figure 11 Scanning electron micrograph (SEM) of the sulfur anion-doped manganese dioxide electrode material prepared from manganese dioxide as a precursor obtained in Example 3 of the invention.
[0098] Figure 12 The X-ray powder diffraction pattern (XRD) under the conditions prepared for Example 3 of the present invention. The abscissa is 2θ, unit: degree; the ordinate is intensity. The curves are manganese dioxide and sulfur ion doped manganese dioxide powder.
[0099] From SEM Figure 11 It can be seen that the MnO 2 The flake structure is maintained before and after the vulcanization process, and its particle size can be measured to be about 200-500nm.
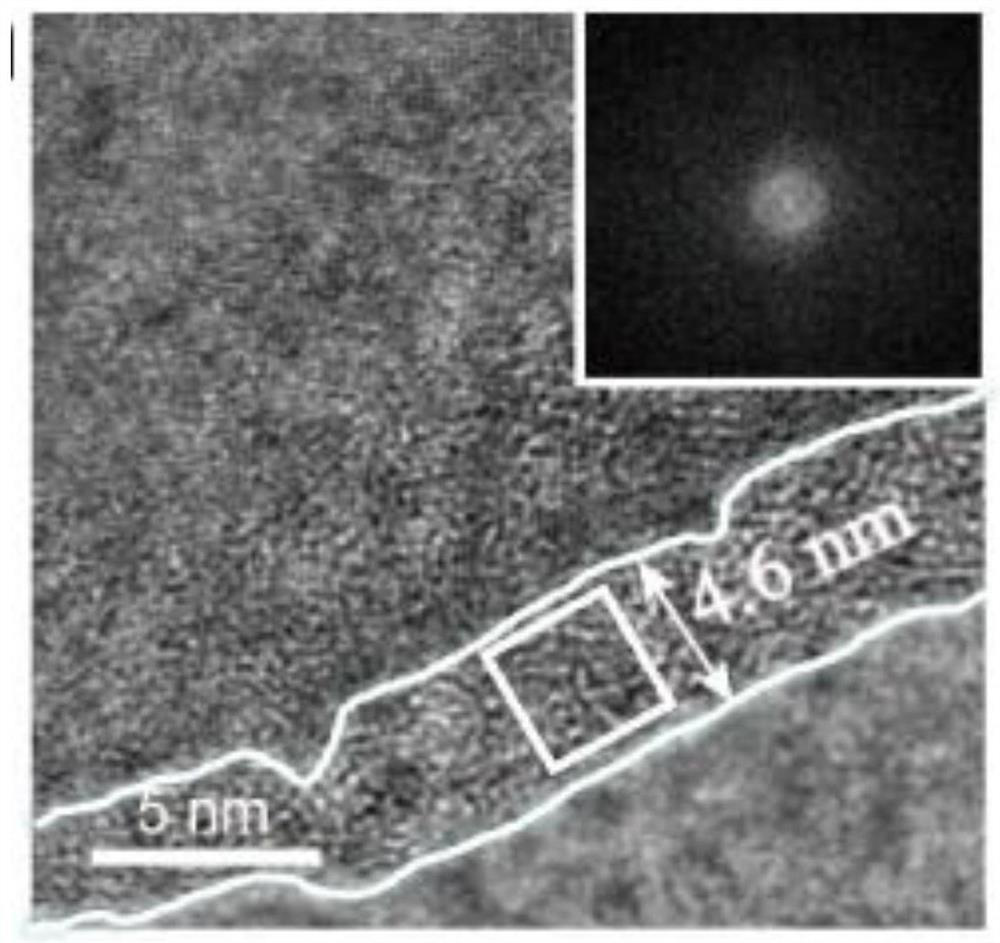
[0100] Figure 15 It is proved th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com