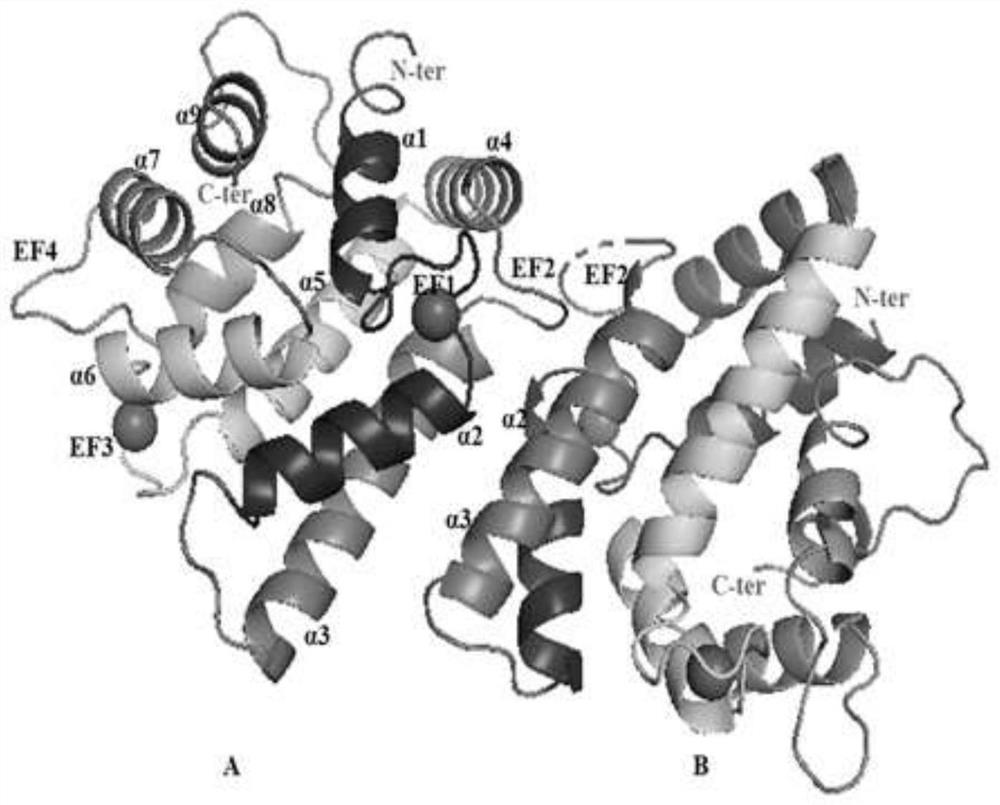
Scapharca subcrenata-derived calcium binding protein ASP-3 with antitumor activity and structure thereof
An ASP-3, calcium binding protein technology, applied in the field of calcium binding protein and its preparation, can solve the problems of inclusion body formation, low efficiency and the like, and achieve the effect of improving anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation method of recombinant clam (Arca subcrenata Lischke, Scapharca subcrenata) calcium-binding protein ASP-3
[0054] 1. Construction of the expression vector of the clam calcium-binding protein ASP-3 gene
[0055] 1.1 Total RNA extraction and cDNA library construction of cockles
[0056] The total RNA of clam was extracted by Trizol method (the clam was purchased from Huangsha Seafood Market, Guangzhou). Put the reagents required for RNA extraction, pipette gun, 1.5mL EP tube and enzyme-free tip into the ultra-clean bench and irradiate it with ultraviolet light for sterilization. Put the cockle samples wrapped in medicine spoon and tinfoil into liquid nitrogen to freeze; add alcohol to the mortar and pestle, sterilize and remove enzymes through high-temperature combustion, and then add liquid nitrogen to the mortar for cooling. The cockle sample was taken out, placed in a mortar, and ground to powder with a pestle. Take 2-3 scoops of sample...
Embodiment 2
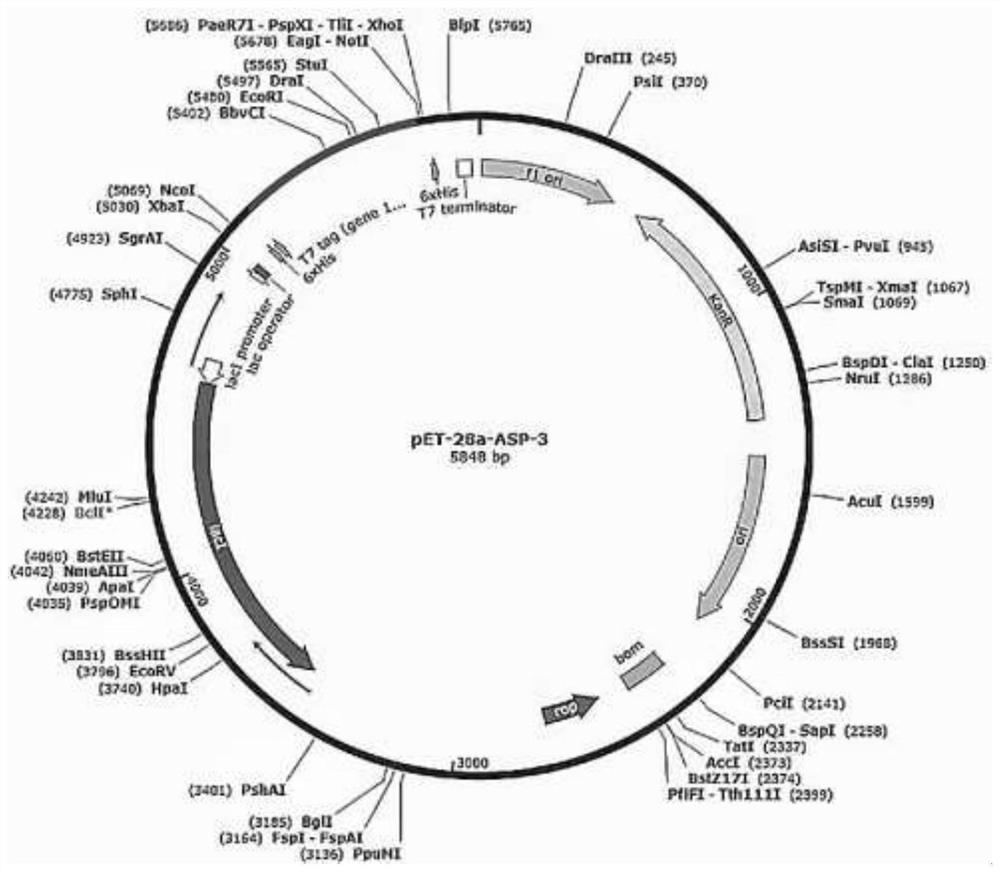
[0113] Example 2 High expression of clam calcium-binding protein gene in E.coli BL21(pET-28a(+))
[0114] The pET-28a(+) recombinant plasmid containing the calcium binding protein gene constructed in Example 1 was transformed into the expression host E.coli BL21(DE3) to obtain the recombinant strain E.coli BL21(DE3). Spread on the LB medium plate containing 50mg / L kanamycin, and pick the positive transformants. Positive clones were cultured overnight at 37°C in 50 mL of LB liquid medium containing 50 mg / L kanamycin, and expanded to 500 mL of LB liquid medium containing 50 mg / L kanamycin at a ratio of 1:100. OD 600 Add IPTG inducer between 0.5-0.6 to a concentration of 0.5mM, and continue culturing at 37°C for 14h. Collect bacteria with LE Buffer (pH=7.4, 20mM Na 2 HPO 4 , 500mM NaCl) after resuspension, the bacterial cells were disrupted by ultrasonic treatment, centrifuged at 13000rpm for 30min at 4°C, and the supernatant was collected, which was the crude protein solutio...
Embodiment 3
[0117] Example 3 Screening of expression conditions in the preparation process of recombinant clam calcium-binding protein ASP-3
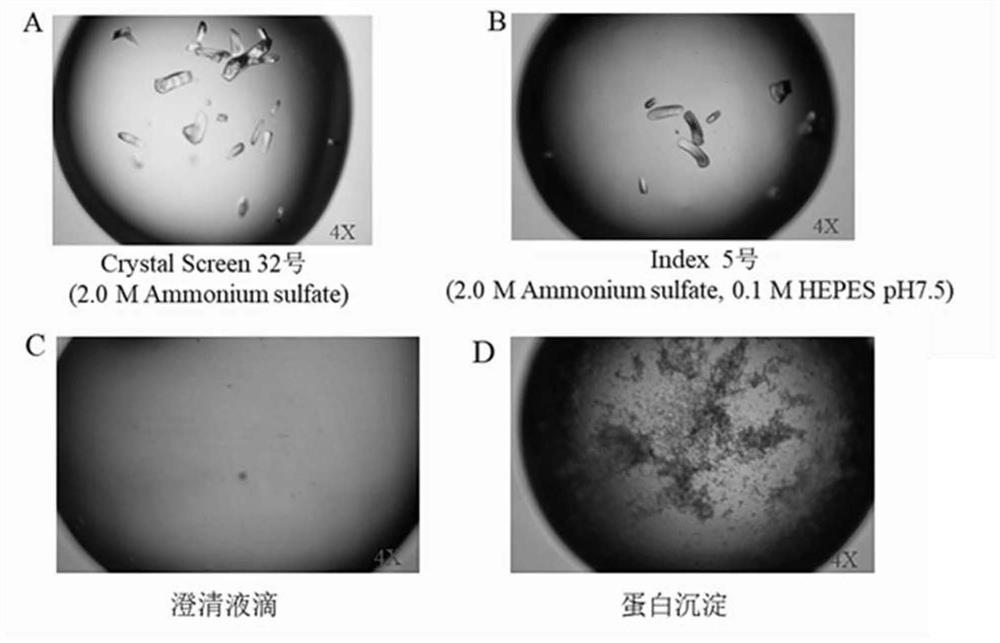
[0118] The specific steps are the same as those shown in Example 2. The screening scheme for expression conditions is shown in Table 9. The expression of ASP-3 under different concentrations of IPTG inducers, different temperatures, and different expression times was investigated, as shown in Table 9.
[0119] Table 9 ASP-3 crystallization condition optimization table
[0120]
[0121]
[0122] Conclusion: When no IPTG inducer is added, there is no expression of ASP-3 protein. When IPTG inducer is added to a concentration of 0.2, the expression of ASP-3 protein is small. When IPTG inducer is added to a concentration of 1.0mM, there are inclusion bodies When the IPTG inducer was added to a concentration of 0.5-0.8mM, the expression of ASP-3 protein was more and no inclusion body was formed, and when the final concentration of IPTG inducer was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com