Anti-cancer drug as well as preparation method and specific application thereof
An anti-cancer drug and drug technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., can solve the problems of immature preparation of chiral nanostructures and insufficient bioavailability, and achieve good pharmaceutical properties, safe and effective side effects, and avoidance of side effects. effect of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Engineered chiral polypeptide-derived exosomes and preparation method
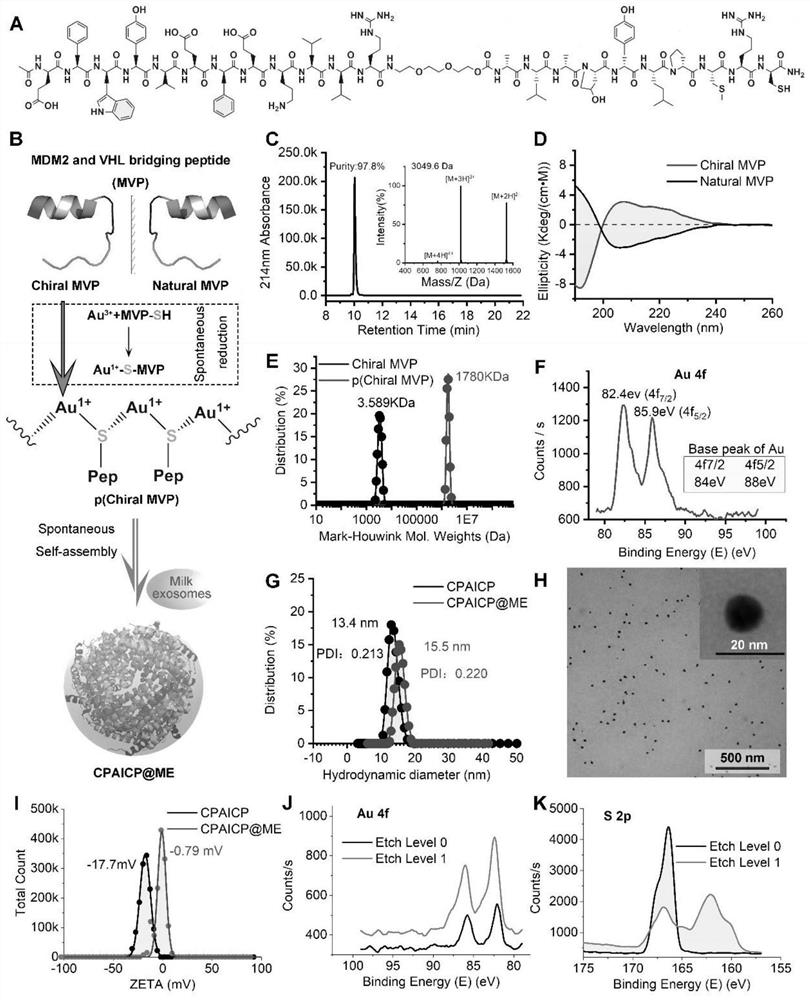
[0076] This example uses a p53 activator - D-peptide, which can specifically degrade MDM2, the most important p53 negative regulatory protein in cancer cells. Such as figure 1 As shown in A, the chiral polypeptide of this example is named chiral MVP (MDM2 and VHL bridging peptide).
[0077] In this example, MVP is a targeted chimeric protein consisting of three functional parts: a dextro-dodecamer motif that binds to MDM2 with high affinity D MBP (MDM2 bind D-peptide), a flexible tripolyethylene glycol linker chain, and a motif VHL (Von Hippel Lindau factor) that binds to the E3 ubiquitin convener.
[0078] In this example, in order to couple Au 3+ With the D-peptide, an additional sulfhydryl chiral Cys residue and a hydrophilic chiral Arg residue were introduced at the C-terminus of the chiral MVP.
[0079] In this embodiment, compared with the natural MVP constructed, D The motif o...
Embodiment 2
[0083] Example 2: Preparation method of engineered chiral polypeptide-derived exosomes
[0084] The difference from Example 1 is that in this example, the D-peptide is replaced with a polypeptide molecule targeting inhibition of beta-catenin, a polypeptide molecule targeting activation of p53 ~ a polypeptide molecule targeting inhibition of PD-L1, etc. Polypeptide molecules of disease-causing proteins.
[0085] In this example, bovine milk exosomes were replaced with exosomes extracted from human bone marrow stem cells, mesenchymal stem cells, umbilical cord stem cells or edible plant extracts.
[0086] Example 2: Stability verification experiment of engineered chiral polypeptide-derived exosome drug
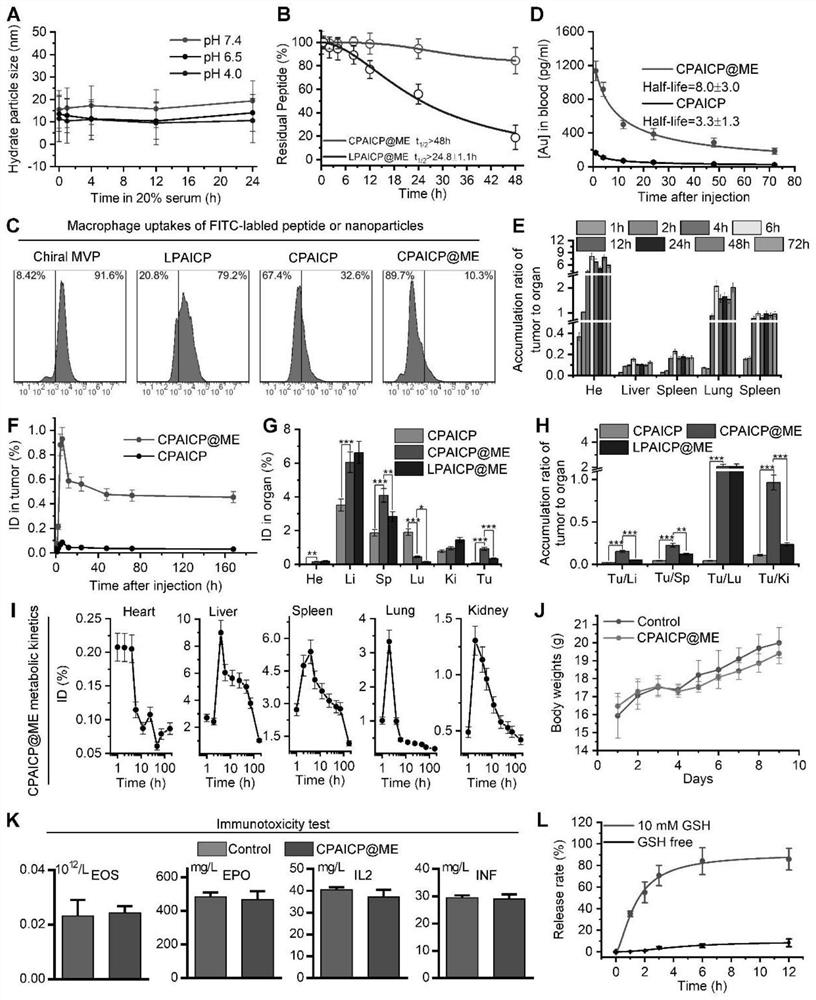
[0087] After the engineered chiral polypeptide-derived exosomes were constructed, their colloidal stability at neutral and acidic pH values was tested through this example. Such as figure 2 As shown in A, the engineered chiral peptide-derived exosomes remained monodisperse...
Embodiment 3
[0088] Example 3: Oral administration of engineered chiral polypeptide-derived exosomes to verify the tumor accumulation effect
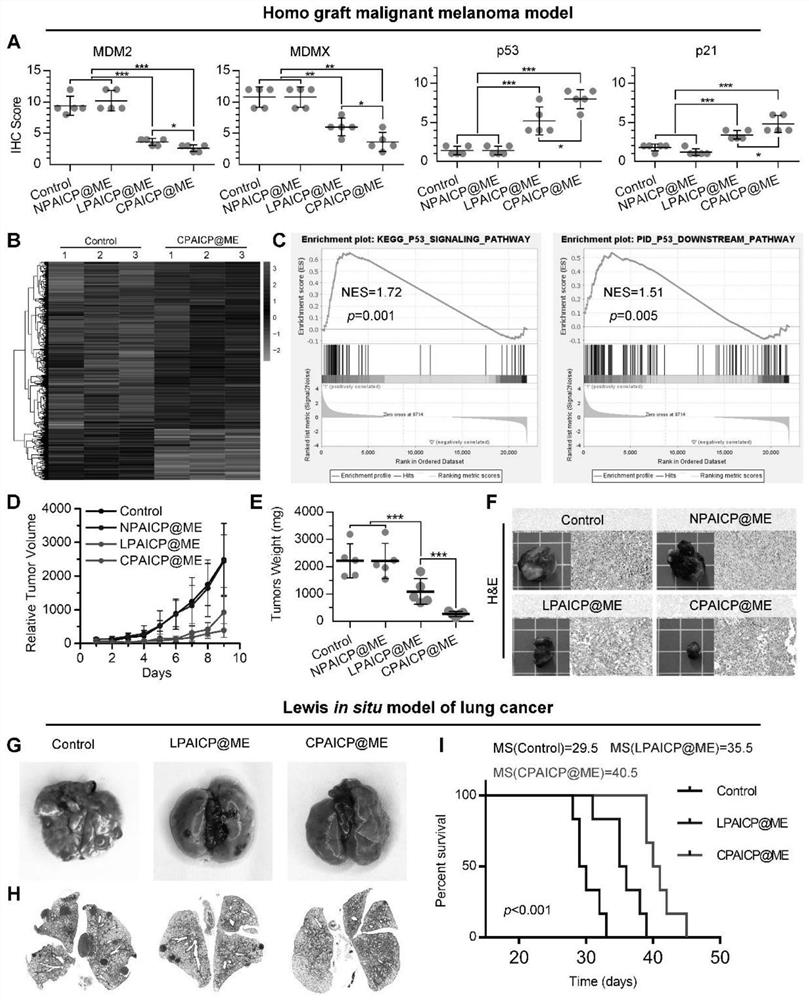
[0089] In this example, in order to verify tumor targeting, 5×10 5 tumor cells to establish a B16F10 melanoma homograft mouse model. After oral administration of engineered chiral polypeptide-derived exosomes or CPAICP at a dose of 2 mg / Kg, ICP-MS was used to analyze the 197 Au was quantified. Such as figure 2 As shown in E, the time-dependent accumulation ratio of engineered chiral polypeptide-derived exosomes in tumors versus normal organs or tissues shows a trend towards tumor accumulation. More importantly, the tumor accumulation of engineered chiral peptide-derived exosomes was nearly ten times that of CPAICP without ME ( figure 2 F). Furthermore, although engineered chiral peptide-derived exosomes, CPAICP and LPAICP@ME exhibit liver, spleen, kidney, and lung accumulation, engineered chiral peptide-derived exosomes increased tumor accumu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com