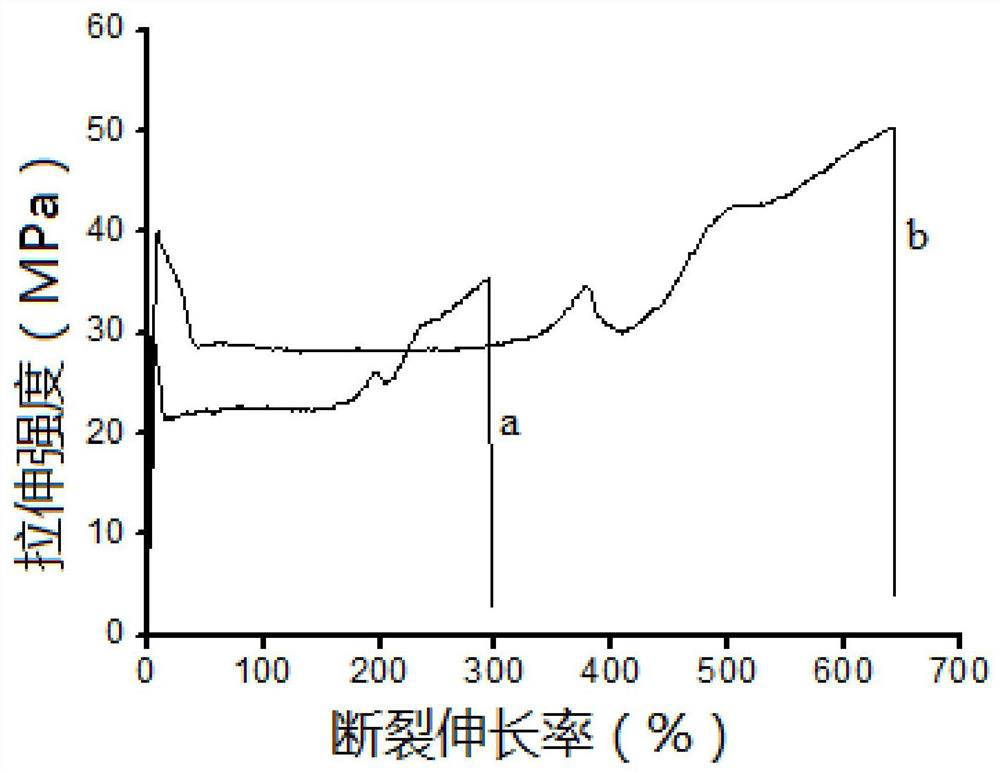
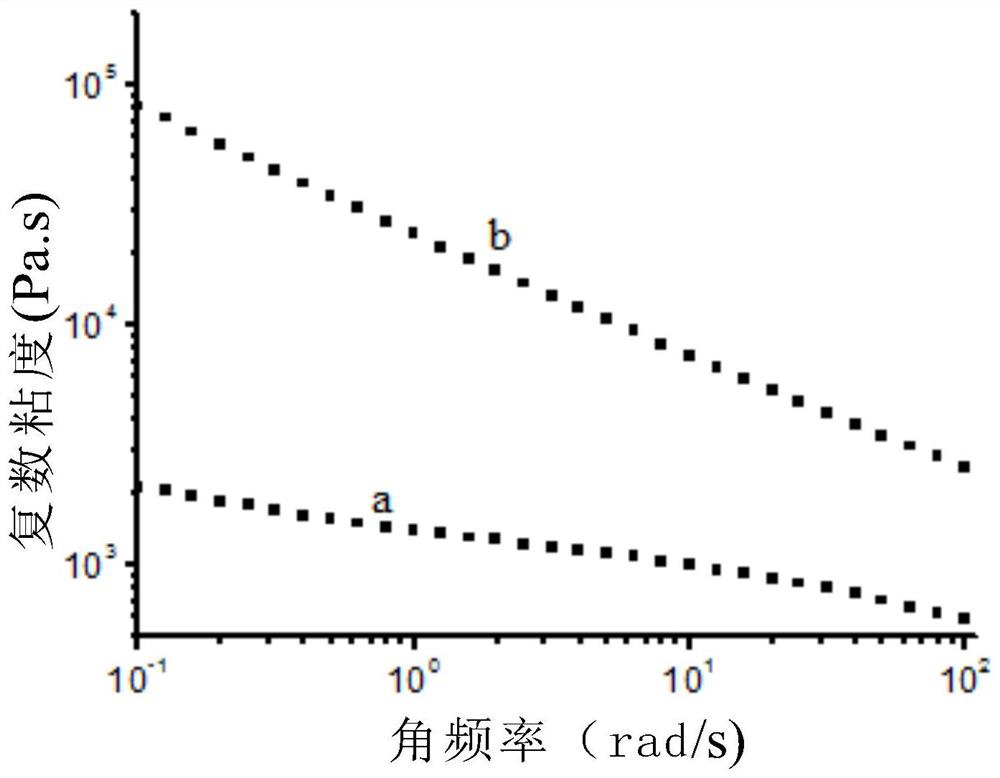
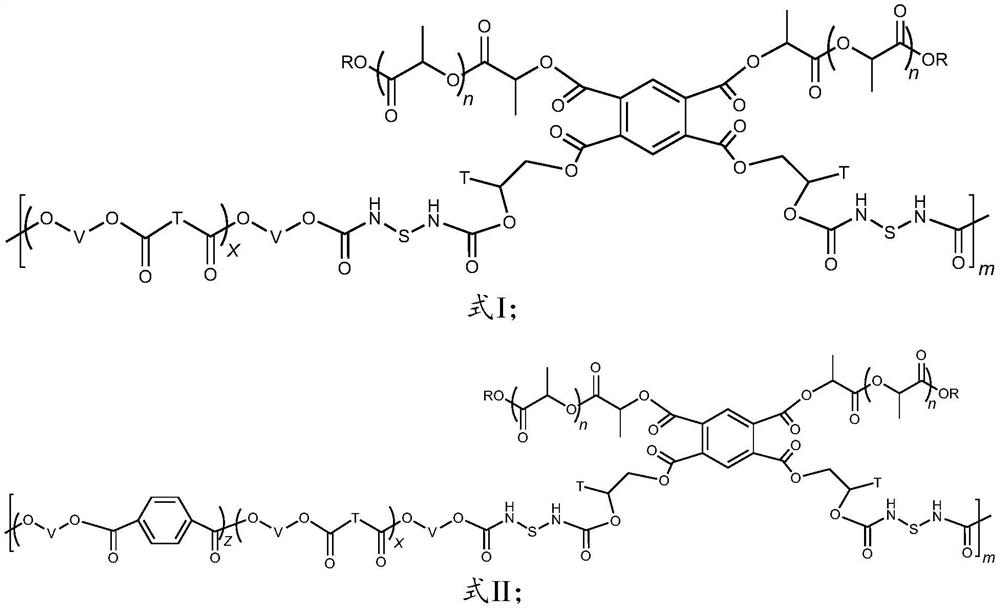
Long-chain-chain polylactic acid-based copolymer and preparation method thereof
A technology of branched polylactic acid-based copolymers, which is applied in the field of long-chain branched polylactic acid-based copolymers and its preparation, can solve the problem of difficulty in obtaining high-molecular-weight polylactic acid copolymers, the inability to effectively improve the melt strength of products, and the difficulty Film applications and other issues, to achieve the effect of process simplification, good biodegradability, and high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The invention provides a method for preparing long-chain branched polylactic acid-based copolymers, comprising:
[0061] Carrying out the first reaction of monohydroxy polylactic acid and phthalic dianhydride to obtain dicarboxylic polylactic acid;
[0062] Carrying out the second reaction with the mono-epoxy polymer and the bis-carboxy polylactic acid to obtain bis-hydroxy polylactic acid;
[0063] The polyester and the dihydroxy polylactic acid are subjected to a chain extension reaction under the action of a chain extender to obtain a long-chain branched polylactic acid-based copolymer whose main chain is polyester and side chains are polylactic acid.
[0064] In the present invention, the monohydroxy polylactic acid is preferably one or more of L-polylactic acid and D-polylactic acid.
[0065] In the present invention, the number average molecular weight of the monohydroxy polylactic acid is preferably 10,000 to 50,000, more preferably 20,000 to 40,000, most prefer...
Embodiment 1
[0087] 1.1 Add 1140g of terephthalic acid, 1340g of adipic acid, 3000g of butanediol and 5g of tetrabutyl titanate into the flask in sequence, then rapidly raise the temperature to 190°C, and when no more liquid distills out of the reaction, raise the temperature to 240°C, The polycondensation reaction was carried out under vacuum at a pressure of 500 Pa. After 6 hours, a poly(butylene adipate-co-butylene terephthalate) copolymer was obtained with a number average molecular weight of 15,000.
[0088] 1.2 Repeated vacuuming and nitrogen filling Cool a 3L round-bottomed flask with a branch pipe, fill it with nitrogen, add 6g of isopropanol, 1440g of L-lactide and 2g of stannous octoate, and react at 130°C. After 15 hours of reaction, heat up Vacuumize to 180°C to remove unreacted monomers, and the pressure is 100Pa to obtain monohydroxy polylactic acid with a number average molecular weight of 16,000.
[0089] 1.3 React 160g of monohydroxy polylactic acid in 1.2 with 1.09g of py...
Embodiment 2
[0096] 2.1 After adding 1140g of terephthalic acid, 1340g of adipic acid, 2000g of ethylene glycol and 5g of tetrabutyl titanate into the flask in sequence, the temperature was rapidly raised to 180°C, and when no liquid distilled out of the reaction, the temperature was raised to 240°C. The polycondensation reaction was carried out under vacuum at a pressure of 500 Pa. After 7 hours, a poly(ethylene adipate-co-ethylene terephthalate) copolymer was obtained with a number average molecular weight of 17,000.
[0097] 2.2 React 200g of monohydroxy polylactic acid prepared in 1.2 of Example 1 with 1.09g of pyromellitic dianhydride at 170°C, and stir the reactant. After reacting for 2 hours, add 1.3g of allyl glycidyl ether , continue to stir, after 2h, obtain bishydroxy polylactic acid; Then add poly(ethylene adipate-co-ethylene terephthalate) and 6g hexamethylene diisocyanate in 150g 2.1 and stir for 15min, The long-chain branched polylactic acid-based copolymer was obtained, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com