Method for recycling discharged water through carbon neutralization
A carbon neutralization and solution technology, which is applied in chemical instruments and methods, separation methods, water/sewage treatment, etc., can solve the problems of solution pH value and conductivity increase, high processing cost, and inability to meet industrial production requirements, etc. Achieve the effect of reducing the conductivity of the solution, meeting the requirements of the reuse index, and solving technical drawbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
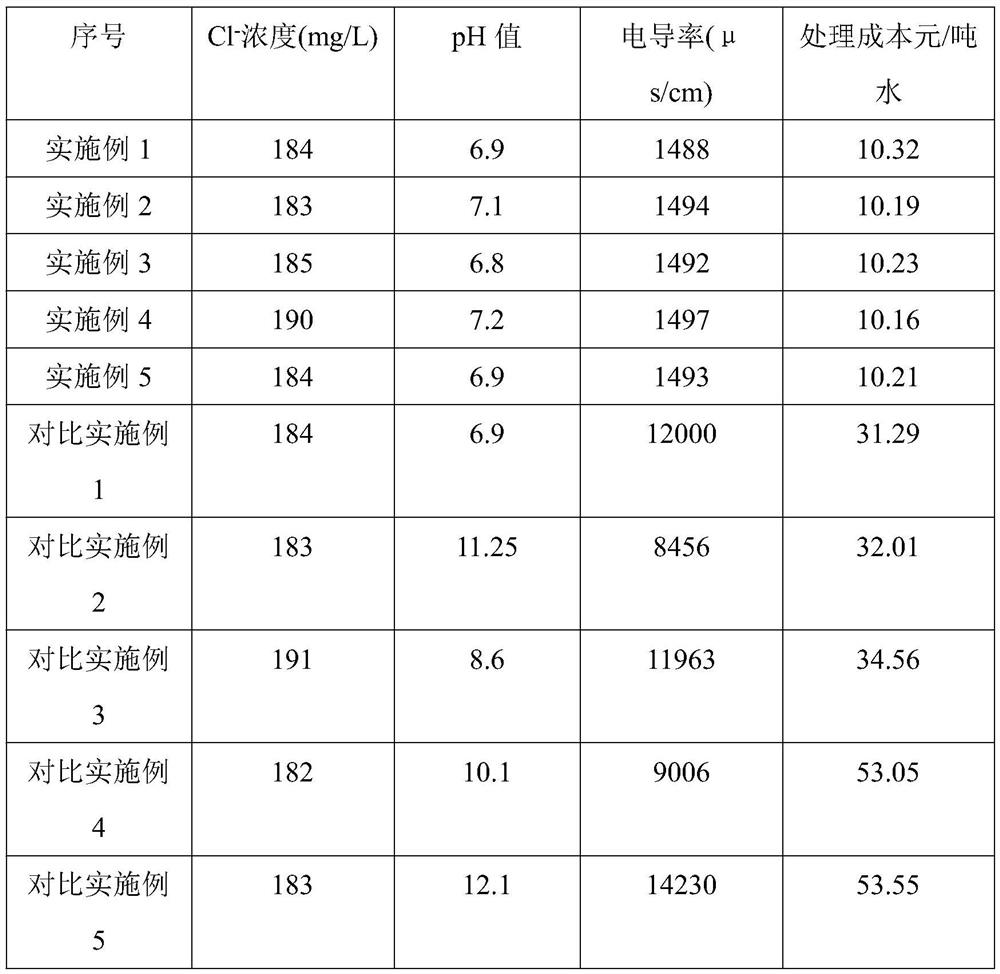
Embodiment 1
[0039] Measure 100ml of external drainage into the reactor, slowly raise the temperature to 25°C, start stirring, and control the stirring speed at 380rpm / min. Add 0.62 g of calcium hydroxide slurry with a mass fraction of 60%, and react at a constant temperature of 25° C. for 30 minutes. After the reaction, the solution was filtered by vacuum filtration, and the filtrate was poured into the reactor again. Add 0.56g of calcium hydroxide and 0.30g of calcium metaaluminate mixed powder into the above reactor, and the initial reaction temperature and stirring speed remain unchanged. After constant temperature reaction for 20 minutes, adjust the stirring speed to 300 rpm / min, continue the reaction for 20 minutes, filter, and transfer the clear liquid into a pressure-resistant three-necked bottle. CO2 gas is introduced through the bottom, and the pressure is controlled at 0.05Mpa. N2 was injected above the three-neck bottle for supplementary pressure, so that the CO2 escape rate ...
Embodiment 2
[0041] Measure 100ml of external drainage into the reactor, slowly raise the temperature to 25°C, start stirring, and control the stirring speed at 380rpm / min. Add 0.50 g of calcium oxide slurry with a mass fraction of 55%, and react at a constant temperature of 25° C. for 30 minutes. After the reaction, the solution was filtered by vacuum filtration, and the filtrate was poured into the reactor again. Add 0.56g of calcium hydroxide and 0.30g of calcium metaaluminate mixed powder into the above reactor, and the initial reaction temperature and stirring speed remain unchanged. After constant temperature reaction for 20 minutes, adjust the stirring speed to 300 rpm / min, continue the reaction for 20 minutes, filter, and transfer the clear liquid into a pressure-resistant three-necked bottle. CO2 gas is introduced through the bottom, and the pressure is controlled at 0.05Mpa. N2 was injected above the three-neck bottle for supplementary pressure, so that the CO2 escape rate was ...
Embodiment 3
[0043]Measure 100ml of external drainage into the reactor, slowly raise the temperature to 25°C, start stirring, and control the stirring speed at 380rpm / min. Add 0.50 g of calcium oxide slurry with a mass fraction of 55%, and react at a constant temperature of 25° C. for 30 minutes. After the reaction, the solution was filtered by vacuum filtration, and the filtrate was poured into the reactor again. Add 0.43g of calcium oxide and 0.30g of calcium metaaluminate mixed powder into the above reactor, and the initial reaction temperature and stirring speed remain unchanged. After constant temperature reaction for 20 minutes, adjust the stirring speed to 300 rpm / min, continue the reaction for 20 minutes, filter, and transfer the clear liquid into a pressure-resistant three-necked bottle. CO2 gas is introduced through the bottom, and the pressure is controlled at 0.05Mpa. N2 was injected above the three-neck bottle for supplementary pressure, so that the CO2 escape rate was 50 bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com