Direct vat set fermentation inoculant for relieving constipation-type irritable bowel syndrome and preparation method and application thereof
A technology for irritable bowel syndrome and fermentative inoculum, applied in microorganism-based methods, biochemical equipment and methods, bacteria used in food preparation, etc., can solve problems such as side effects, constipation recurrence, etc. Growth and stability-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
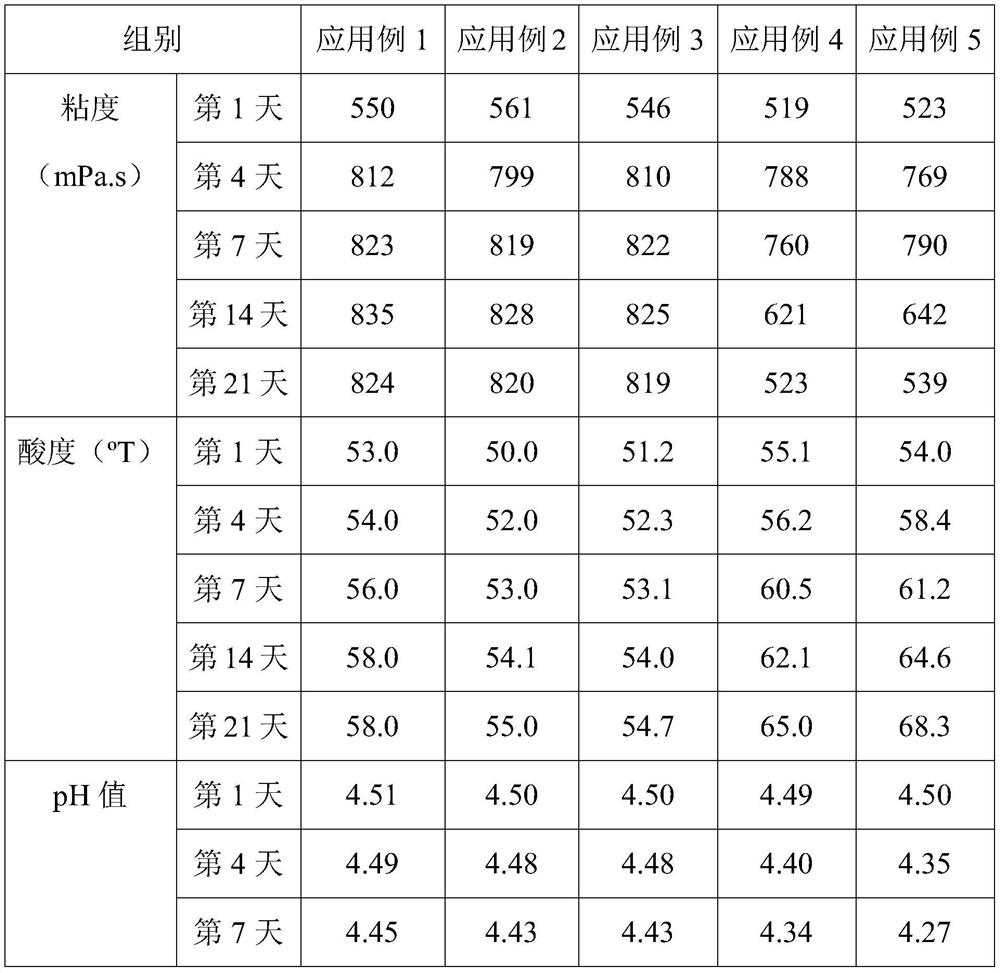
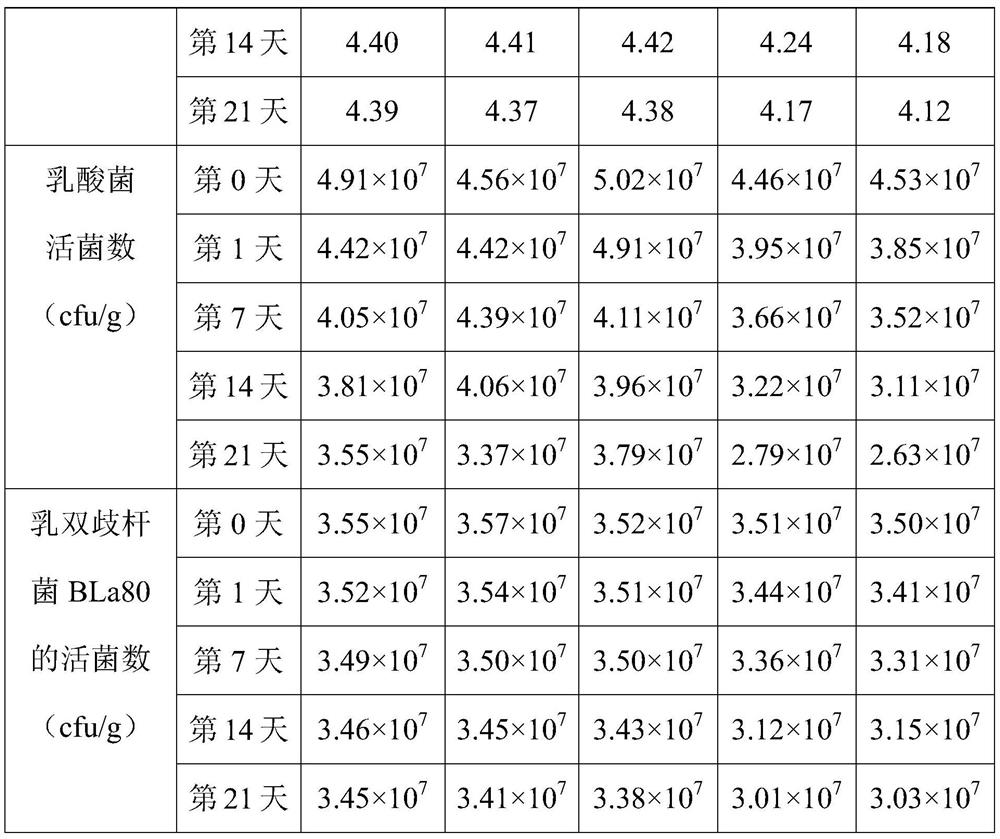
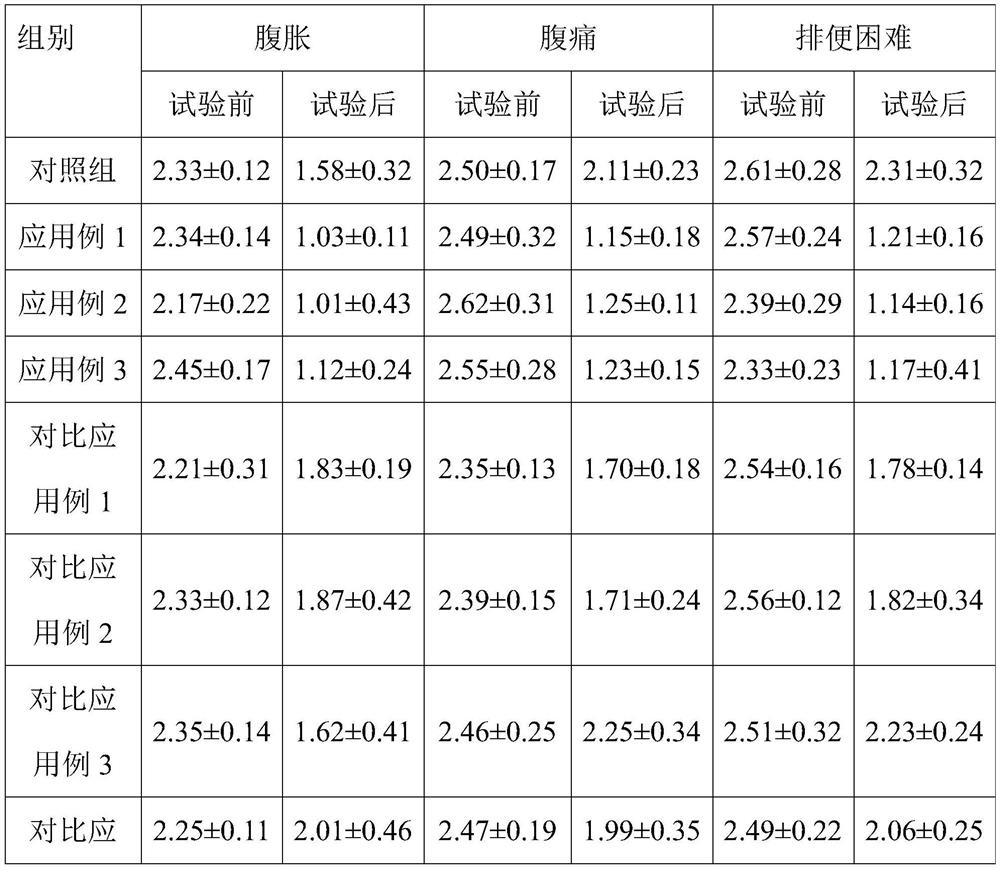
[0067] This example provides a direct-injection fermented bacterial agent for alleviating constipation-type irritable bowel syndrome, which includes 30 parts of Streptococcus thermophilus ST81 fermented product, 1.5 parts of Lactobacillus bulgaricus LB42 fermented product, lactobacillus 2.5 parts of Fidobacterium BLa80 fermentation product, 12.5 parts of trehalose, 4 parts of maltodextrin, 1 part of β-cyclodextrin, 3 parts of sucrose, 1.5 parts of glycerin, 0.05 parts of mannitol and 0.2 parts of Tween-80. Among them, the number of live bacteria of Bifidobacterium lactis BLa80 in the direct-injection fermented bacterial agent for relieving constipation-type irritable bowel syndrome was 1×10 11 cfu / g.
[0068] Its preparation method is as follows:
[0069] (1) Inoculate the Bifidobacterium lactis BLa80 preserved in the glycerol tube in the MRS liquid medium at a ratio of 2%, culture it statically at 37°C for 22 hours, and activate it for 3 generations. The activated seeds wer...
Embodiment 2
[0074] This example provides a direct-injection fermented bacterial agent for alleviating constipation-type irritable bowel syndrome, which includes 25 parts of Streptococcus thermophilus ST81 fermented product, 2 parts of Lactobacillus bulgaricus LB42 fermented product, lactobacillus 2.5 parts of Mycobacterium BLa80 fermentation product, 14 parts of trehalose, 8 parts of maltodextrin, 1.5 parts of β-cyclodextrin, 2 parts of sucrose, 2.2 parts of glycerin, 0.25 parts of Tween-800 and 0.05 parts of mannitol.
[0075] Its preparation method is as follows:
[0076] (1) Streptococcus thermophilus ST81 stored in glycerol tubes was inoculated into MRS liquid medium at a ratio of 2%, cultured statically at 37°C for 22 hours, and activated for 3 generations. Inoculate the activated seeds into a fermenter with 2% inoculum, incubate at pH 5.5, 37°C for 11 hours, collect the bacterial solution and centrifuge at 10,000 r / min for 10 minutes, discard the supernatant, collect the bacterial s...
Embodiment 3
[0081] This example provides a direct-injection fermented bacterial agent for alleviating constipation-type irritable bowel syndrome, which includes 32 parts of Streptococcus thermophilus ST81 fermented product, 1.5 parts of Lactobacillus bulgaricus LB42 fermented product, lactobacillus 2.5 parts of Mycobacterium BLa80 fermentation product, 13 parts of trehalose, 3 parts of maltodextrin, 1.5 parts of β-cyclodextrin, 2 parts of sucrose, 0.01 part of mannitol, 1 part of glycerol and 0.2 parts of Tween-80.
[0082] Its preparation method is as follows:
[0083] (1) Lactobacillus bulgaricus LB42 preserved in glycerol tubes was inoculated in MRS liquid culture medium at a ratio of 2%, cultured statically at 37°C for 22 hours, and activated for 3 generations. Inoculate the activated seeds into a fermenter with 2% inoculum, incubate at pH 5.5, 37°C for 11 hours, collect the bacterial solution and centrifuge at 10,000 r / min for 10 minutes, discard the supernatant, collect the bacteria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com