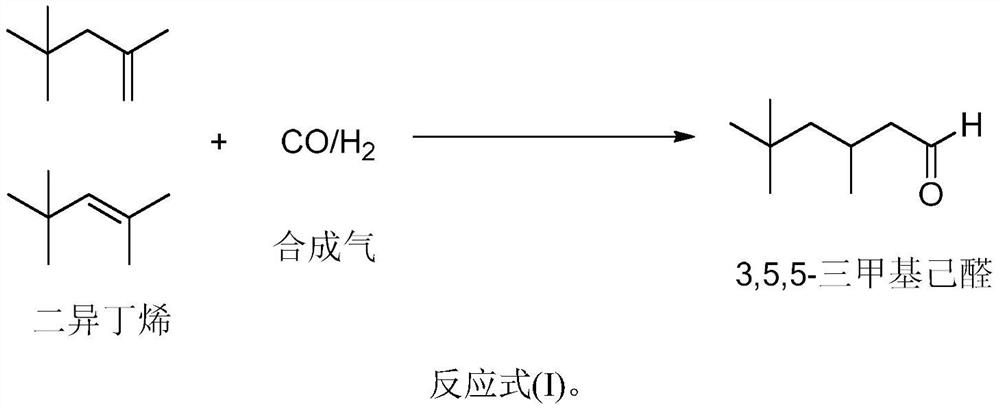
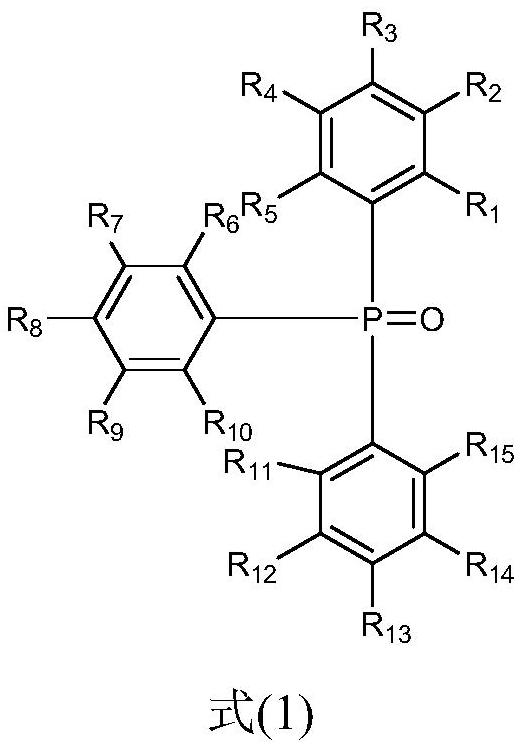
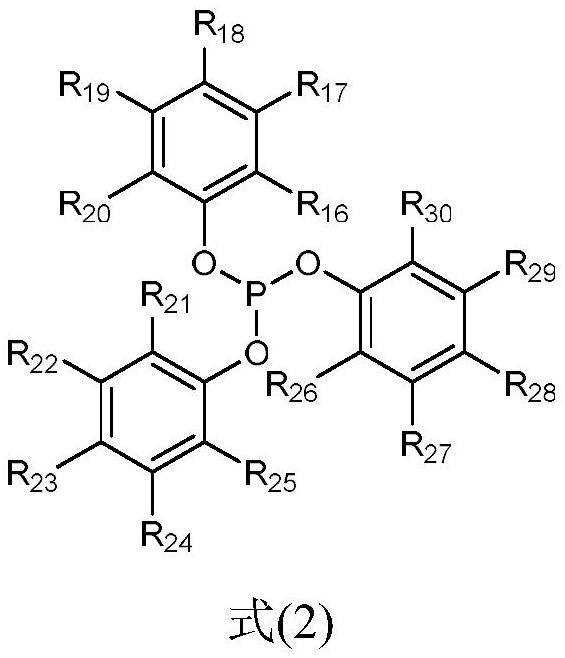
Synthesis method of 3, 5, 5-trimethylhexanal, catalytic system and application
A technology of trimethylhexanal and a synthesis method, which is applied in the field of homogeneous catalysis, can solve the problems of harsh reaction conditions, low activity of a catalytic system, instability, etc., and achieves the effects of stabilizing the catalytic system, improving the reaction activity, and being difficult to deactivate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of synthetic method of 3,5,5-trimethylhexanal, the method comprises the following steps:
[0028] In the presence of metal catalysts, triphenylphosphine oxide compound 1 (L1), and organic phosphine compound 2 (L2), diisobutene undergoes hydroformylation reaction with synthesis gas to obtain the 3,5,5-trimethylhexyl aldehyde.
[0029]
[0030] In the method of the present invention, the metal catalyst is a metal rhodium compound, including but not limited to rhodium trichloride, rhodium acetate, carbonylbis(triphenylphosphino)rhodium chloride, rhodium dicarbonyl acetylacetonate, 1,5-cyclooctyl Diene (acetylacetonate) rhodium, (acetylacetonyl) carbonyl (triphenylphosphine) rhodium, hydrogenated carbonyl tris (triphenylphosphine) rhodium, bis (triphenylphosphine) carbonyl rhodium chloride, tris (triphenylphosphine) rhodium One or more of rhodium chloride, tetrakis(triphenylphosphine)rhodium hydride, etc. Preferably, the metal catalyst is ...
Embodiment 1
[0101]
[0102] Weigh 2.58mg (0.01mmol) of rhodium dicarbonyl acetylacetonate catalyst, 307.47mg (0.5mmol) of three (2,4-di-tert-butylphenyl) phosphine oxide, 129.4mg (0.2mmol) of three (2, 4-di-tert-butylphenyl) phosphite, a certain amount of toluene solvent is placed in a 100ml autoclave, after using nitrogen replacement three times, the diisobutylene raw material (2 , the molar ratio of 4,4-trimethyl-1-pentene to 2,4,4-trimethyl-2-pentene is 7:3), and then replaced by a mixture of carbon monoxide and hydrogen (1:1) 5 times, then pour in the mixed gas of carbon monoxide and hydrogen (1:1) to 3MPa, the rhodium concentration in the reaction system is 100ppm, start stirring and then heat the reactor to 110°C, after 5 hours of constant temperature and pressure reaction, the reactor will naturally Cooled to room temperature, evacuated the remaining gas in the reactor under ventilated conditions, opened the reactor, added an internal standard to take a sample, analyzed the prod...
Embodiment 2
[0104] Using the same operating method as in Example 1 to react, compared with Example 1, three (2,4-di-tert-butylphenyl) phosphine oxide was replaced by 268.35mg (0.5mmol) of three (2-(tert-butyl base)-4-methoxyphenyl)phosphine oxide, the total conversion of diisobutene was 96%, and the yield of 3,5,5-trimethylhexanal was 91%.
[0105]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com