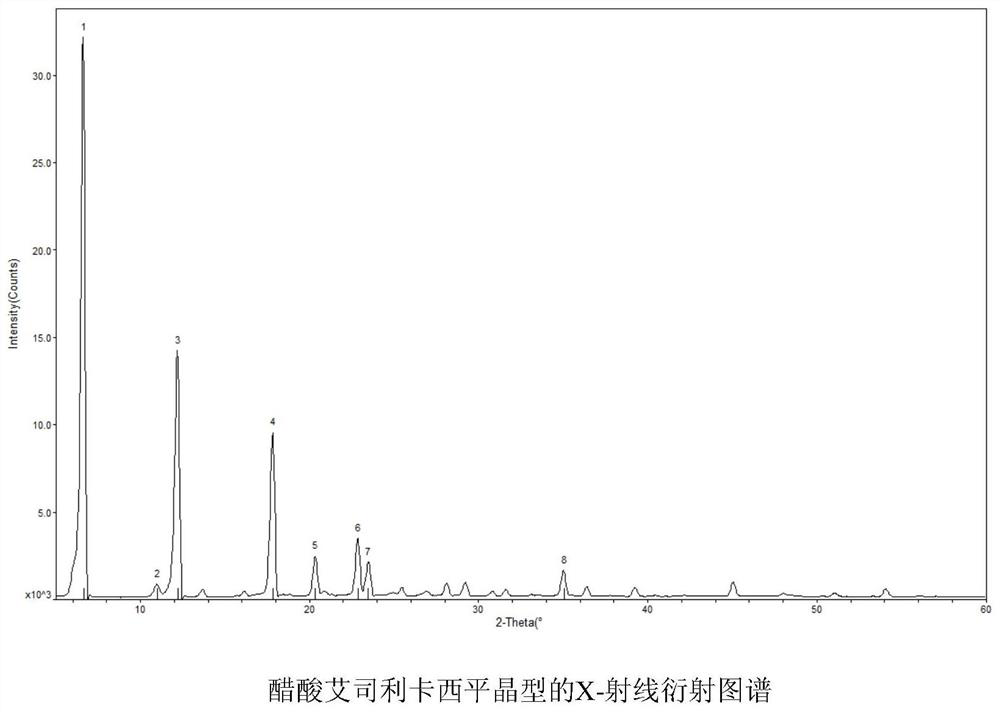
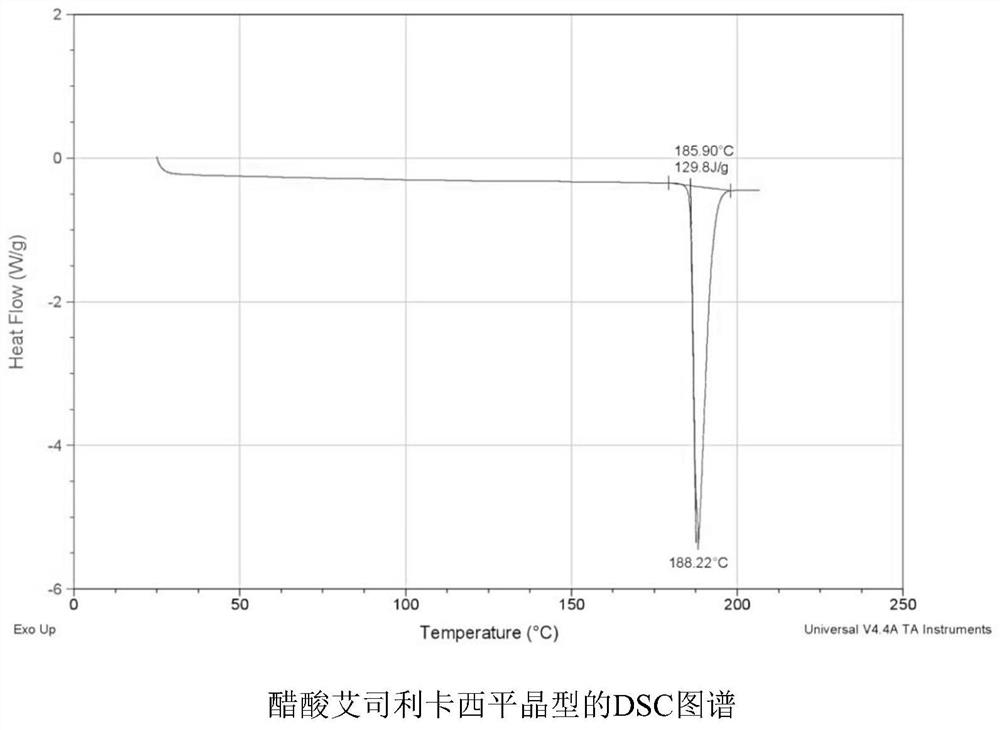
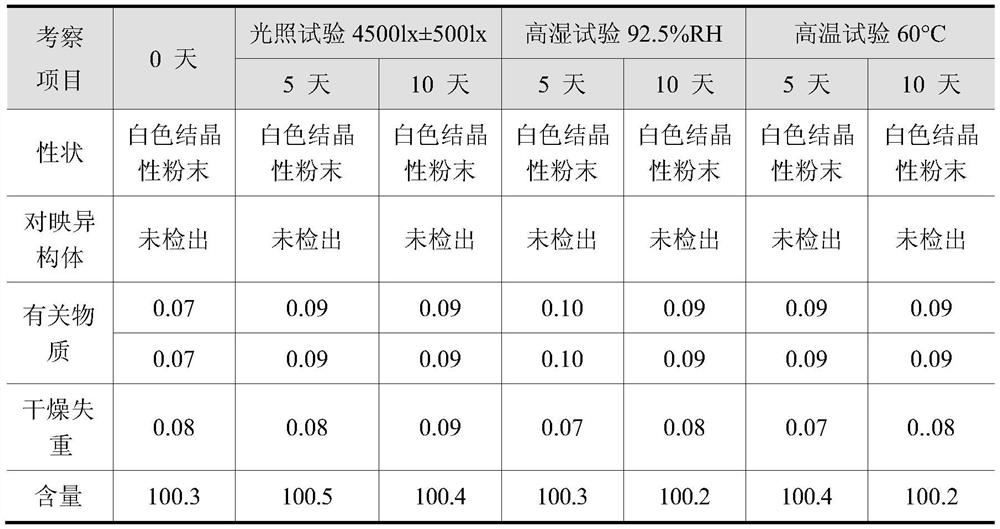
Synthesis method of eslicarbazepine acetate with high purity and stable crystal form
A technology of eslicarbazepine acetate and crystal form, which is applied to the synthesis method of high-purity eslicarbazepine acetate and the field of stable crystal forms thereof, can solve the problem that industrialized production cannot be realized, heavy metal residues in the final product, and by-products are difficult to remove and other problems, to achieve the effects of good chemical stability, low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Take 43g of 10-methoxyiminostilbene, add 101g of tetrahydrofuran, add 72g of hydrochloric acid, heat to 60-65°C, react for 5h, add about 258g of tap water, stir for 10min, and suction filter to obtain a yellow sandy solid in the middle Body I is about 38g, and the yield is 95%.
Embodiment 2
[0032] Example 2: Take 25g of intermediate I, add dichloromethane, lower the temperature by 5-10°C, and add BH dropwise 2 .Me 2 S 95.g and R-2-Me-CBS 33g, stirred for 3h, added 405g of hydrochloric acid, separated, and the organic phase was concentrated under reduced pressure to obtain 29g of intermediate II with a yield of 75%.
Embodiment 3
[0033] Example 3: Take 25g of intermediate I, add dichloromethane, lower the temperature by 5-10°C, and add BH dropwise 2 .Me 2 85.g of S and 28g of R-2-Me-CBS were stirred for 3h, 405g of hydrochloric acid was added, the layers were separated, and the organic phase was concentrated under reduced pressure to obtain 27g of intermediate II with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com