Detection kit for detecting lung cancer immune checkpoints by protein chip technology
An immune checkpoint and protein chip technology is applied in the field of immune checkpoint detection kits for detecting lung cancer by protein chip technology, which can solve the problems of not prolonging the PFS of patients, increasing the incidence of adverse reactions, and accelerating the malignant process of patients, and achieves stability and reliability. High sensitivity, specificity and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] The present invention will be further described below in conjunction with the accompanying drawings and embodiments.
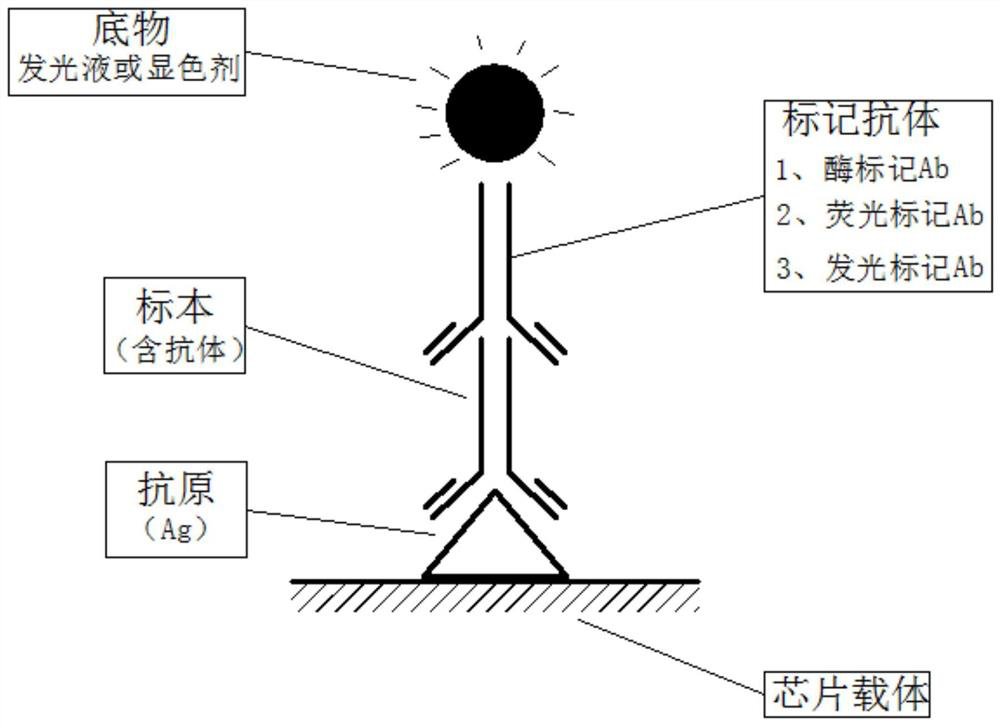
[0017] Such as figure 1 As shown, a protein chip technology detection kit for lung cancer immune checkpoint detection, combined with the background technology compared with the traditional enzyme-linked method, chemiluminescence method, gold standard half-dot method, the protein chip technology detection kit developed by the present invention for lung cancer immune detection kit , which solves the drawbacks of traditional methods that can only identify one protein at a time, and can detect the serum status of multiple antibodies with only one experiment. It has the advantages of time-saving, high-efficiency, and high-throughput parallel detection. The crystallization of blood technology, and the test results can not only be observed qualitatively with the naked eye, but also can be equipped with a reader for quantitative detection. The results can be st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com