Synthesis method of 4-hydroxy-2, 2, 6-trimethyl cyclohexanone
A technique for the synthesis of trimethylcyclohexanone, which is applied in the field of organic synthesis, can solve problems such as high difficulty in synthesis, difficulty in synthesis, and limited sources, and achieve the effects of reducing the difficulty of synthesis, increasing the total yield, and simplifying the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
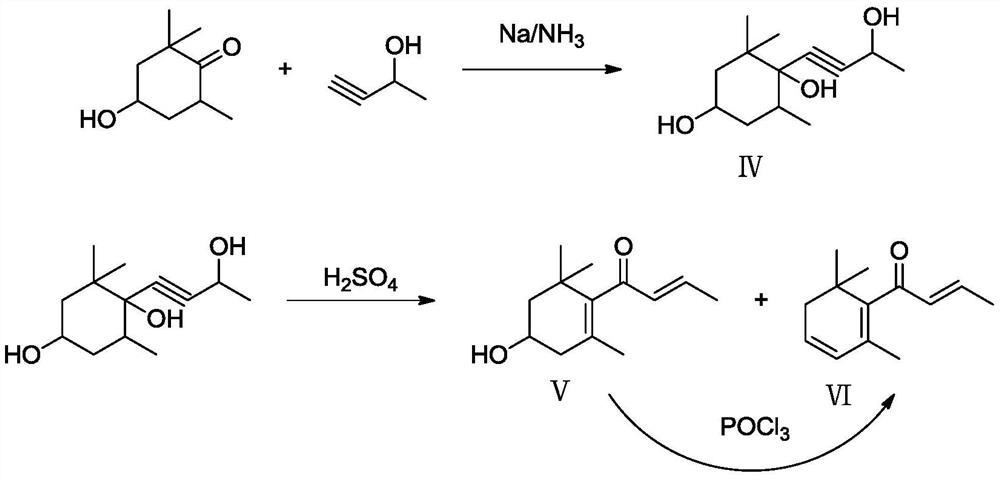
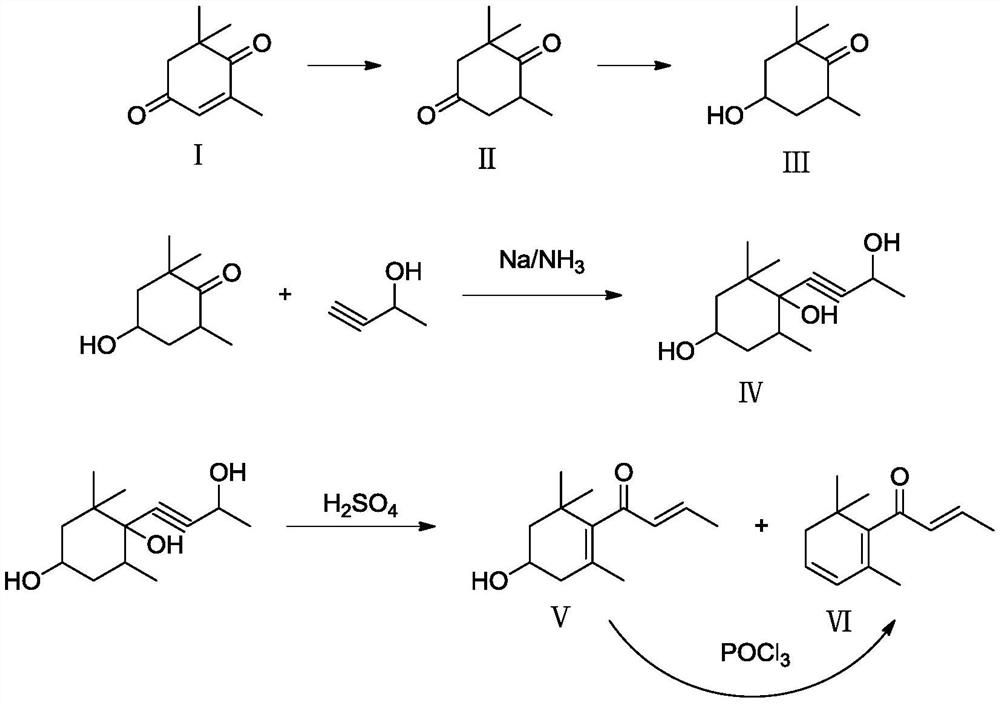
[0032] (1) First, add 1521.2 g of tea aroma ketone, 15.2 g of 5% palladium-calcium carbonate catalyst, and 1500 g of ethanol to the autoclave, seal the autoclave, replace nitrogen for 3 times, press nitrogen to 3.0 MPa and confirm that the autoclave is well sealed , evacuate the nitrogen, replace with 2.0MPa hydrogen for 6 times, turn on the stirring paddle, keep the hydrogen pressure at 2.0MPa, and keep the inner temperature of the reactor at 60°C for 3h. After the stirring was stopped and the gas was released, the reaction liquid was analyzed by GC, and the composition of the reaction liquid was: 99.72% of 2,2,6-trimethyl-1,4-cyclohexanedione, 0.05% of tea aroma ketone, and 0.23% of others. The reaction solution was filtered, and ethanol was distilled off under reduced pressure at 90°C and 100 hPa to obtain 1543.3 g of crude 2,2,6-trimethyl-1,4-cyclohexanedione.
[0033] (2) Under an inert atmosphere, firstly mix 0.5mmol Rh(CO) 2 Dissolve acac in 5mL absolute ethanol, then ...
Embodiment 2
[0036](1) First, add 1521.2 g of tea aroma ketone, 8.0 g of 10% palladium-calcium carbonate catalyst, and 800 g of ethanol to the autoclave, seal the autoclave, replace nitrogen 3 times, press nitrogen to 6.0 MPa after confirming that the autoclave is well sealed , evacuate the nitrogen, replace with 5.0MPa hydrogen for 6 times, turn on the stirring paddle, keep the hydrogen pressure at 5.0MPa, and keep the inner temperature of the reactor at 70°C for 5 hours. After the stirring was stopped and the gas was released, the reaction liquid was analyzed by GC, and the composition of the reaction liquid was: 99.82% of 2,2,6-trimethyl-1,4-cyclohexanedione, 0.02% of tea aroma ketone, and 0.16% of others. The reaction solution was filtered, and ethanol was distilled off under reduced pressure at 90°C and 100 hPa to obtain 1543.5 g of crude 2,2,6-trimethyl-1,4-cyclohexanedione.
[0037] (2) Under an inert atmosphere, firstly mix 0.025mmol Rh 4 (CO) 12 Dissolve in 5mL absolute ethanol,...
Embodiment 3
[0040] (1) first add tea aroma ketone 1521.2g, 10% palladium-calcium carbonate catalyst 80g, ethanol 1000g to autoclave, autoclave is sealed, after nitrogen replacement 3 times, nitrogen stamping to 2.0MPa confirms that autoclave is well sealed, Evacuate the nitrogen, replace it with 1.0MPa hydrogen for 6 times, turn on the stirring paddle, keep the hydrogen pressure at 1.0MPa, and keep the inner temperature of the reactor at 80°C for 12 hours. After the stirring was stopped and the gas was released, the reaction liquid was analyzed by GC, and the composition of the reaction liquid was: 99.43% of 2,2,6-trimethyl-1,4-cyclohexanedione, 0.01% of tea aroma ketone, and 0.56% of others. The reaction solution was filtered, and ethanol was distilled off under reduced pressure at 90°C and 100 hPa to obtain 1543.6 g of crude 2,2,6-trimethyl-1,4-cyclohexanedione.
[0041] (2) Under an inert atmosphere, firstly mix 0.15mmol Rh 6 (CO) 16 Dissolve in 5mL absolute ethanol, then dissolve 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com