Purification method of loratadine key intermediate
A technology of loratadine and a purification method, which is applied in the field of purification of key intermediates of loratadine, can solve the problems of difficult preparation of loratadine, many by-products, low purity, etc., to ensure process stability, Improvement of quality and yield, high yield and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A method for purifying the key intermediate of loratadine, comprising:
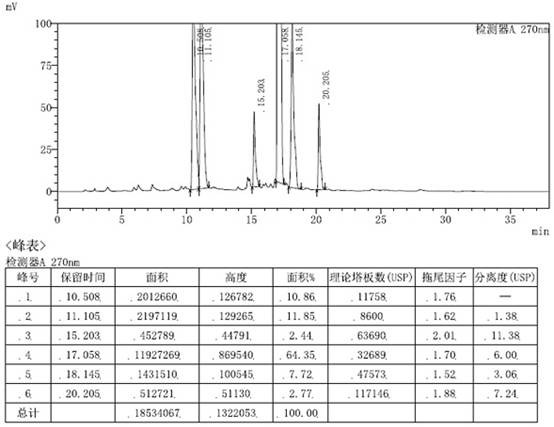
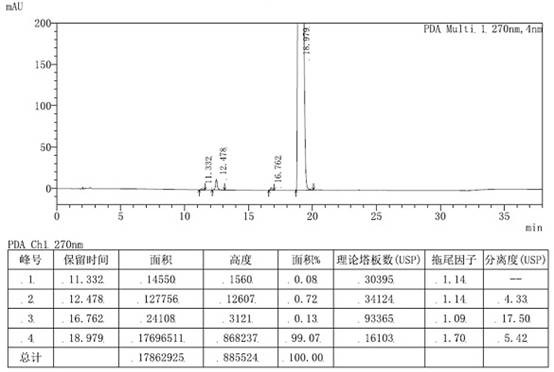
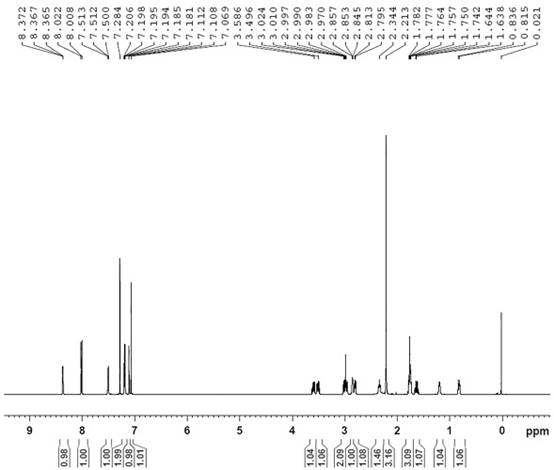
[0050]Add 10.00 g (0.029 mol) of the crude intermediate of loratadine into 60 mL of ethanol, heat to 60-70 °C and stir to dissolve, add 3.39 g (0.029 mol) of fumaric acid, keep stirring for 1 hour, cool down to 0-10 ℃, stirred for 1 hour, filtered the solid with suction, added the obtained solid to 200mL 5% sodium hydroxide aqueous solution, added 200mL ethyl acetate, extracted, separated liquids, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure until no liquid was dropped, Add 45mL of isopropyl acetate, heat to dissolve, cool down and crystallize to obtain 5.42g of the purified loratadine key intermediate, with a yield of 54.2% and a purity of 99.07% as measured by HPLC. The HPLC chromatogram is as figure 2 Shown, NMR structure confirmation see image 3 shown.
[0051] Add 40.0g of concentrated sulfuric acid into the reaction flask, start stirring, coo...
Embodiment 2
[0053] A method for purifying the key intermediate of loratadine, comprising:
[0054] Add 10.00 g (0.029 mol) of the crude intermediate of loratadine into 60 mL of ethanol, heat to 60-70 °C and stir to dissolve, add 3.39 g (0.029 mol) of fumaric acid, keep stirring for 1 hour, cool down to 0-10 ℃, stirred for 1 hour; filtered the solid with suction, added 200mL of 5% sodium hydroxide aqueous solution to the obtained solid, added 200mL of ethyl acetate, extracted, separated liquids, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure until no liquid was dropped, Add 45mL of ethyl acetate, heat to dissolve, cool down to crystallize, and obtain 3.46g of purified loratadine key intermediate, with a yield of 34.6% and a purity of 99.10% as measured by HPLC.
Embodiment 3
[0056] A method for purifying the key intermediate of loratadine, comprising:
[0057] Add 10.00 g (0.029 mol) of the crude intermediate of loratadine into 60 mL of ethanol, heat to 60-70 °C and stir to dissolve, add 3.39 g (0.029 mol) of fumaric acid, keep stirring for 1 hour, cool down to 0-10 ℃, stirred for 1 hour; filtered the solid with suction, added 200mL of 5% sodium hydroxide aqueous solution to the obtained solid, added 200mL of ethyl acetate, extracted, separated liquids, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure until no liquid was dropped, Add 150 mL of methyl tert-butyl ether, heat, cool down and crystallize to obtain 7.01 g of the purified loratadine key intermediate, with a yield of 70.1% and a purity of 78.48% as measured by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com