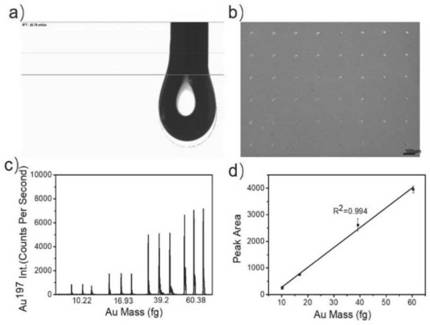
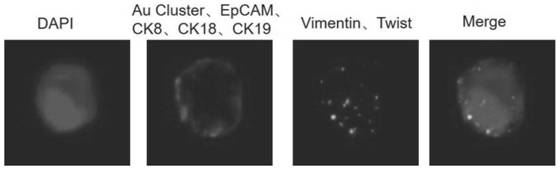
Polypeptide-metal cluster probe for specifically recognizing CTC membrane protein and application of polypeptide-metal cluster probe for quantitatively detecting expression quantity of membrane protein
A metal cluster and specific technology, applied in the field of medical detection, can solve the problems of inability to enrich CTCs with purity, inability to quantitatively analyze marker proteins, insufficient specificity, etc., and achieve simple preparation steps, less reagents, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The invention provides a preparation method of the polypeptide-metal cluster probe, comprising the following steps:
[0052] 1) drop the metal source solution into the targeting polypeptide solution and mix to obtain a polypeptide-metal source mixed solution;
[0053] 2) performing a reduction reaction on the metal source in the polypeptide-metal source mixture in step 1), and the formed metal atoms form clusters and bind to the targeted polypeptide to obtain polypeptide-metal clusters.
[0054] In the invention, the metal source solution is dropped into the targeted polypeptide solution and mixed to obtain a polypeptide-metal source mixed solution.
[0055] In the present invention, there is no special limitation on the type of the metal source, and metal sources well known in the art can be used. In the embodiment of the present invention, the metal source is HAuCl 4 solution. The concentration of the metal source solution is preferably 20-30 mM, more preferably 25...
Embodiment 1
[0067] (1) In this embodiment, the polypeptide-gold cluster 1 is used, and the targeting peptide is a polypeptide targeting the cell membrane protein N-cadherin, and the sequence is: H 2 N-SWTLYTPSGQSKKKKKGYCC-COOH (Peptide1), the specific preparation steps are as follows:
[0068] Prepare a clean 5mL small glass bottle and a magnet with a diameter of 1cm. Dissolve 2mg of peptide dry powder in 596μL of ultrapure water, slowly add it dropwise into the small glass bottle with the magnet, and stir at 400rpm / min. 3min. 36 μL of 25 mM HAuCl 4 The solution was slowly added dropwise to the above solution and stirred thoroughly for 3 min. Add 179 μL of 0.5M NaOH solution dropwise to the above system, and continue to stir for 5 minutes, then wrap the above system with tinfoil paper and place it on a magnetic stirrer with a set temperature of 37°C, and stir at 600rpm / min to avoid Light reaction 26h. After the reaction is completed and cooled to room temperature, the peptide-gold clu...
Embodiment 2
[0080] (1) Using polypeptide-gold cluster 2, the targeting peptide is a polypeptide targeting cell membrane protein N-cadherin, the sequence is: H 2 N-CCYSWTLYTPSGQSKKKKKG-COOH (Peptide2), the specific preparation steps are as follows:
[0081] Prepare a clean 5mL small glass bottle and a magnet with a diameter of 1cm. Dissolve 2mg of peptide dry powder in 596μL of ultrapure water, slowly add it dropwise into the small glass bottle with the magnet, and stir at 400rpm / min. 3min. 36 μL of 25 mM HAuCl 4 The solution was slowly added dropwise to the above solution and stirred thoroughly for 3 min. Add 358 μL of 0.5M NaOH solution dropwise to the above system, and continue to stir for 5 minutes, then wrap the above system with tinfoil paper and place it on a magnetic stirrer with a set temperature of 37°C, and stir at a speed of 600rpm / min to avoid Light reaction 26h. After the reaction is completed, cool down to room temperature to obtain the targeting peptide-modified polypep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com