Synthesis method of molybdenum trichloride
A technology of molybdenum trichloride and synthesis method, applied in the directions of molybdenum halide, tin chloride, tin halide, etc., can solve the problem of unsatisfactory purity of molybdenum trichloride, affecting the purity of molybdenum trichloride, and low yield of molybdenum trichloride and other issues, to achieve the effect of being conducive to maximizing utilization, being environmentally friendly, and having high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
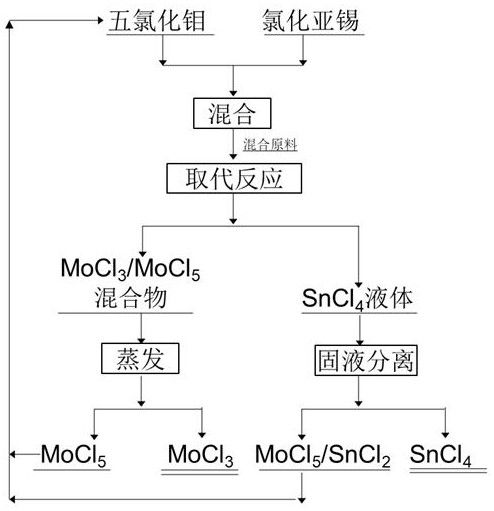
[0028] The molar ratio of molybdenum pentachloride to tin protochloride is 1.2:1, weigh 2.4 mol of molybdenum pentachloride and 2 mol of stannous chloride, mix them and put them into a replacement reactor, and after argon replacement, react after replacement The reactor was kept warm at 120°C, and the rear end of the reactor was cooled to collect tin tetrachloride liquid. After 4 hours of reaction, the molybdenum pentachloride cooling receiver was exhausted, and the temperature was raised to 250°C. After 4 hours of heat preservation, the reactor was cooled and collected, and three Molybdenum chloride 1.95 mol, purity 99.1%, molybdenum pentachloride 100g collected by cooling, tin tetrachloride liquid filtration obtains tin tetrachloride 1.98 mol, purity 99.4%, filter residue 1g, solid-liquid separation method is centrifugation or filtration, five Molybdenum chloride and filter residue can be reused as raw materials.
Embodiment 2
[0030] The molar ratio of molybdenum pentachloride and tin protochloride is 2:1, weigh 4 mol of molybdenum pentachloride and 2 mol of stannous chloride, mix and put into the replacement reactor, the volume ratio of argon and nitrogen is 1: 1. After the replacement, keep the reactor warm at 140°C, cool the rear end of the reactor to collect the tin tetrachloride liquid, react for 4 hours, exhaust from the molybdenum pentachloride cooling receiver, raise the temperature to 280°C, and keep it warm for 4 hours, The reactor was cooled to collect materials, and 1.98 mol of molybdenum trichloride was obtained with a purity of 99.3%, 540 g of molybdenum pentachloride collected by cooling, and 1.99 mol of tin tetrachloride was obtained by centrifugation of the tin tetrachloride liquid, with a purity of 99.6%, and 1.3 g of centrifuged residue. The solid-liquid separation method is a combination of decantation and gravity sedimentation, and molybdenum pentachloride and centrifugal slag ca...
Embodiment 3
[0032] The molar ratio of molybdenum pentachloride and tin protochloride is 3:1, weigh 6 mol of molybdenum pentachloride and 2 mol of stannous chloride, mix them and put them into a replacement reactor, after argon replacement, react after replacement The reactor was kept warm at 160°C, and the rear end of the reactor was cooled to collect the tin tetrachloride liquid. After 4 hours of reaction, the exhaust was exhausted from the molybdenum pentachloride cooling receiver, and the temperature was raised to 320°C. After 4 hours of heat preservation, the reactor was cooled and collected, and three Molybdenum chloride 1.96 mol, purity 99.5%, molybdenum pentachloride 1100g collected by cooling, tin tetrachloride liquid gravity settling after 1 day, extract supernatant, bottom suspension liquid filter, obtain tin tetrachloride 1.99 mol altogether, purity 99.5%, filter residue 3g, solid-liquid separation method is filtration, molybdenum pentachloride and filter residue can be reused a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com