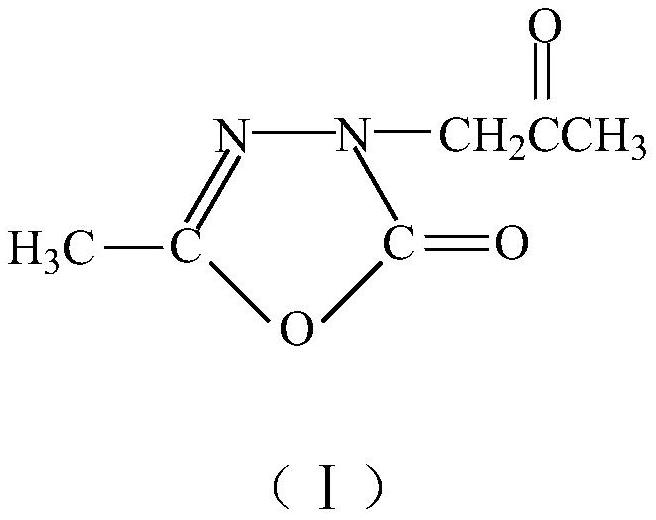
Preparation method of 2, 3-dihydro-5-methyl-2-oxo-1, 3, 4-oxadiazole-3-acetone
A technology of oxadiazole and methyl, which is applied in the field of chemical synthesis of pesticides, can solve the problems of poor feasibility of industrial production, inability to participate in market competition, and many dangerous reaction processes, so as to avoid side reactions, reduce reaction activation energy, and shorten reaction time. the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
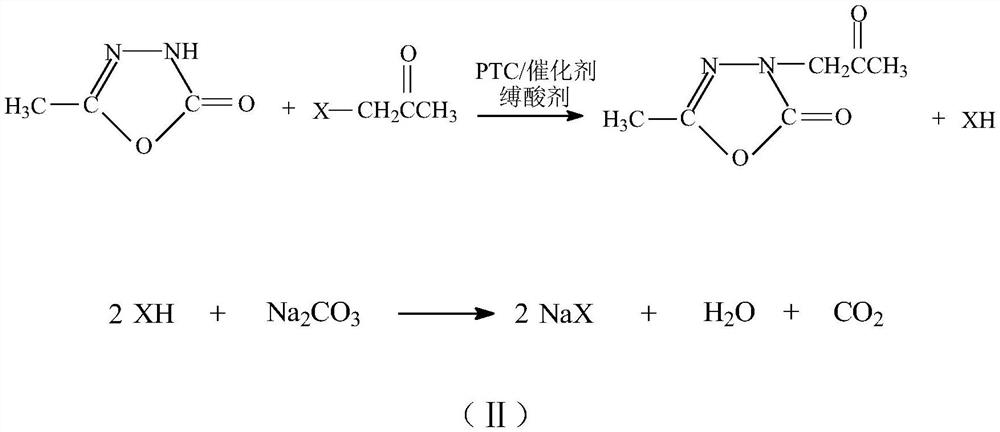
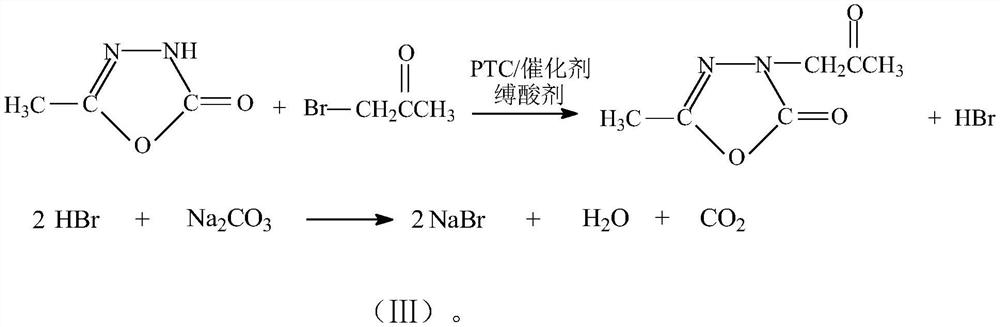
[0038] In the 3000L alkylation reactor, 2500kg of solvent dichloroethane, 1000kg of raw material 5-methyl-1,3,4-oxadiazole-2-(3H)-one, 580kg of acid binding agent sodium carbonate, Transfer catalyst tetrabutylammonium bromide 25kg, catalyst potassium iodide 25kg, start stirring, adopt 70 ℃ of hot water heating to be heated to 45 ℃~50 ℃, and slowly add 917 kg of monochloroacetone dropwise under the insulation, and after the dripping is completed, 55 ℃~ 65 ℃ insulation reaction for 2.5hr, sampling and analysis raw material 5-methyl-1,3,4-oxadiazol-2-(3H)-one residue≤0.80%, monochloroacetone residue≤0.50%, insulation reaction 3.0hr cooling Cool down to 20℃~35℃, discharge the material and filter with airtight suction, filter and wash the solid, the suction filtration operation is relatively easy, the solid inorganic salt is light yellow or light gray white, and the filtrate is a light golden yellow clear solution.
[0039] The filtrate is transferred to a vacuum distillation kettl...
Embodiment 2
[0041] In the 3000L alkylation reactor, add 2000kg of solvent toluene, 1000kg of raw material 5-methyl-1,3,4-oxadiazole-2-(3H)-one, 580kg of acid binding agent sodium carbonate, four phase transfer catalysts 25kg of butylammonium bromide, 15kg of catalyst sodium iodide, start stirring, adopt 70 ℃ of hot water to heat up to 45 ℃~50 ℃, and slowly add 917kg of monochloroacetone dropwise under the insulation, after the dropwise addition is completed, 55 ℃~65 ℃ ℃ heat preservation reaction for 3.0hr, sample and analyze raw material 5-methyl-1,3,4-oxadiazol-2-(3H)-one residual 1.8%, monochloroacetone residual about 1.3%, continue to heat preservation reaction for 1.0hr, sample If the analysis is qualified, cool down to 20℃~35℃, discharge the material in a closed suction filtration, filter and wash the solid, the suction filtration operation is relatively easy, the solid inorganic salt is light yellow or light gray white, and the filtrate is a light golden yellow clear solution.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com