Ritonavir impurity and preparation method thereof
A technology for ritonavir and impurities, applied in the field of chemical synthesis, can solve the problems of difficult separation, unknown impurities, unknown reference substances of impurities, etc., and achieves the effects of simple operation and improved yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Tropavir Impact
[0038] The preparation of the ritonavir impurities includes the following steps:
[0039] 20.00 g of Compound I, 133 mL dioxane, 13 ml DMSO, 5.04 g of di-triphenyl phosphorus diphenoid, 0.72 g of Cucl and 21.60 g of copper with copper, nitrogen gas replacement were added to a 500 ml reaction flask, 0.72 g of Cucl and 21.60 g of copper with copper with copper with copper, nitrogen gas replacement. The temperature was warmed to 120 ° C for 1 h; the TLC detection of the feedstock reaction was complete, the reaction liquid decreased to room temperature, and 200 ml of ethyl acetate and 200 ml of water were added to the reaction mixture, and the organic phase was washed with 200 ml of water, collect organic phases, reduce The pressure concentrated to obtain 19.80 g of the oil, 100 ml of ethyl acetate was added to the obtained oil, and the temperature was stirred at 70 ° C and dissolved, then slow down to room temperature, filtered, the f...
Embodiment 2
[0041] Example 2 Preparation of Tropavir Impact
[0042] The preparation of the ritonavir impurities includes the following steps:
[0043] 20.00 g of Compound I, 133 mL dioxane, 13 ml DMSO, 7.04 g of triphenyl phosphorus, 5.50 g of palladium chloride, 0.72 g of Cucl and 21.60 g of copper with copper with copper, and 21.60 g of copper, and nitrogen were replaced 3 times. The reaction liquid was warmed to 120 ° C for 1 h; TLC detection of the feedstock reaction, the reaction liquid decreased to room temperature, and 200 ml of ethyl acetate and 200 ml of water were added to the reaction mixture, and the organic phase was separated, and the organic phase was washed with water. The oil was concentrated under reduced pressure to obtain 19.10 g of the oil, and 90 ml of ethyl acetate was added to the obtained oil, and the temperature was stirred at 70 ° C and dissolved, then slow down to room temperature, filtered, filter cake was washed with EtOAc Ethyl acetate, 50 ° C dry 12H was obtai...
Embodiment 3
[0044] Example 3 Preparation of Tornavir Impact
[0045] The preparation of the ritonavir impurities includes the following steps:
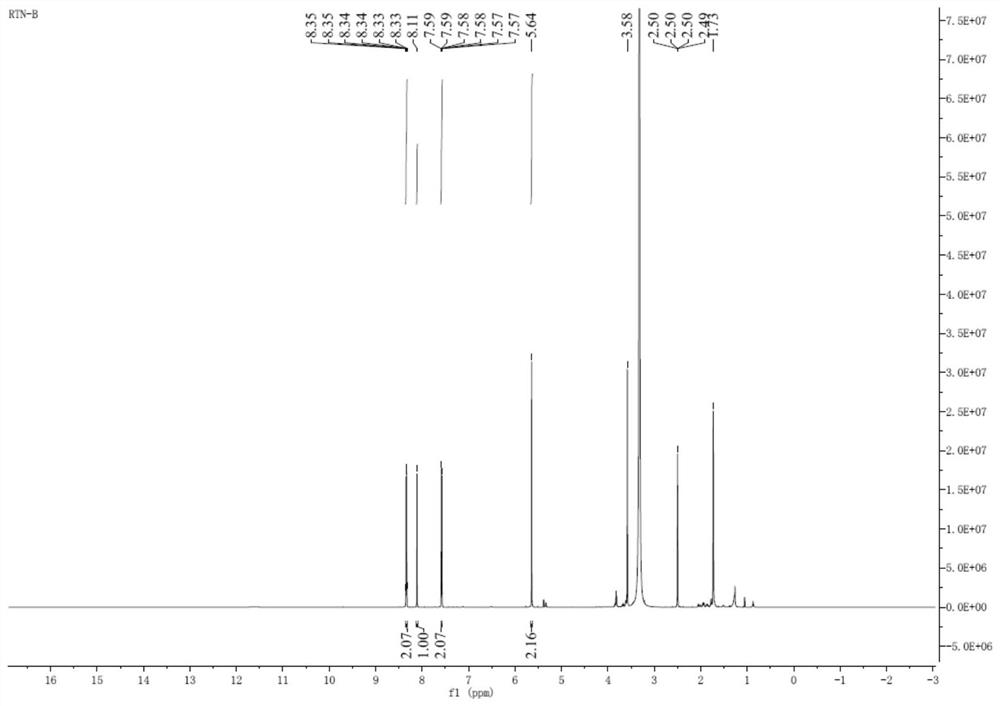
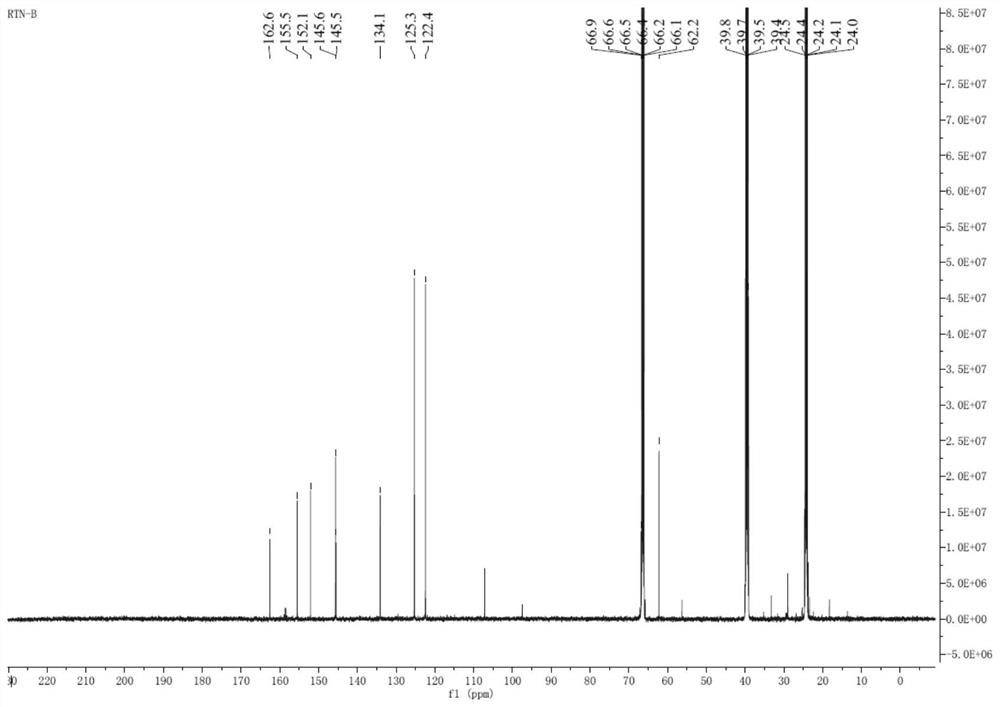
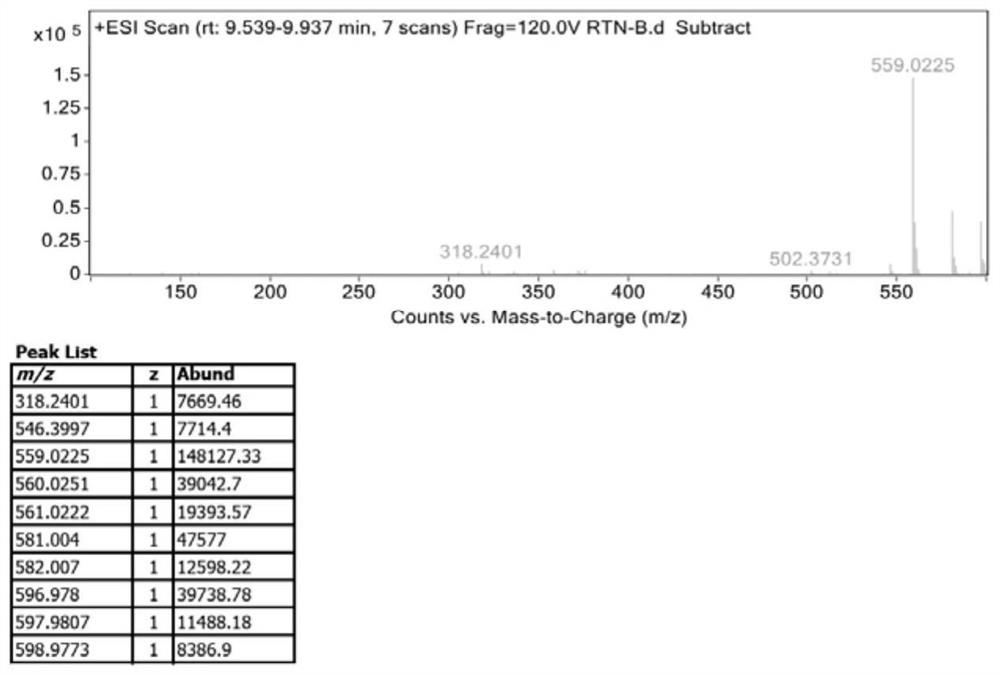
[0046]5.00 g of Compound I, 50 mL DMF, 0.40 g of Palladium, 7.12 g of copper, 5.04 g of potassium fluoride, and 4.54 g of silver nitrate, 5.04 g of potassium fluoride and silver nitrate, 5.54 g of silver nitrate, and the reaction liquid was stirred to 120 ° C. 1H; TLC detection of raw material reactions completely, reaction liquid decreased to room temperature, add 50 ml of ethyl acetate and 50 ml of water to the reaction mixture, divided into organic phases, washed with 50 ml of water, collect organic phase, concentrated under reduced pressure to obtain an oil 4.70 g, add 25 ml of ethyl acetate to the obtained oil, warmed to 70 ° C and stirred complete, then slowly fell to room temperature, filtered, filter cake was washed with EtOAc. 82.5%. The nuclear magnetic characterization data is in the same embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com