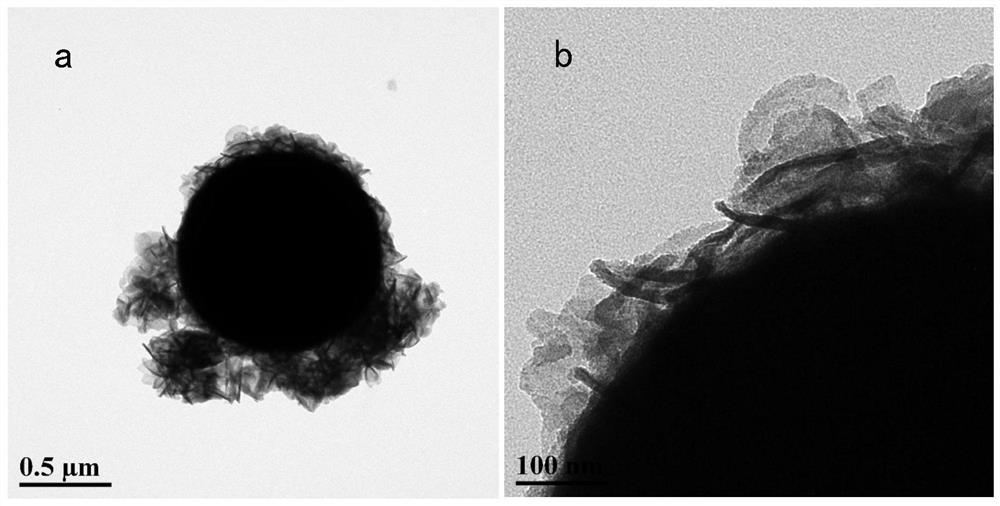
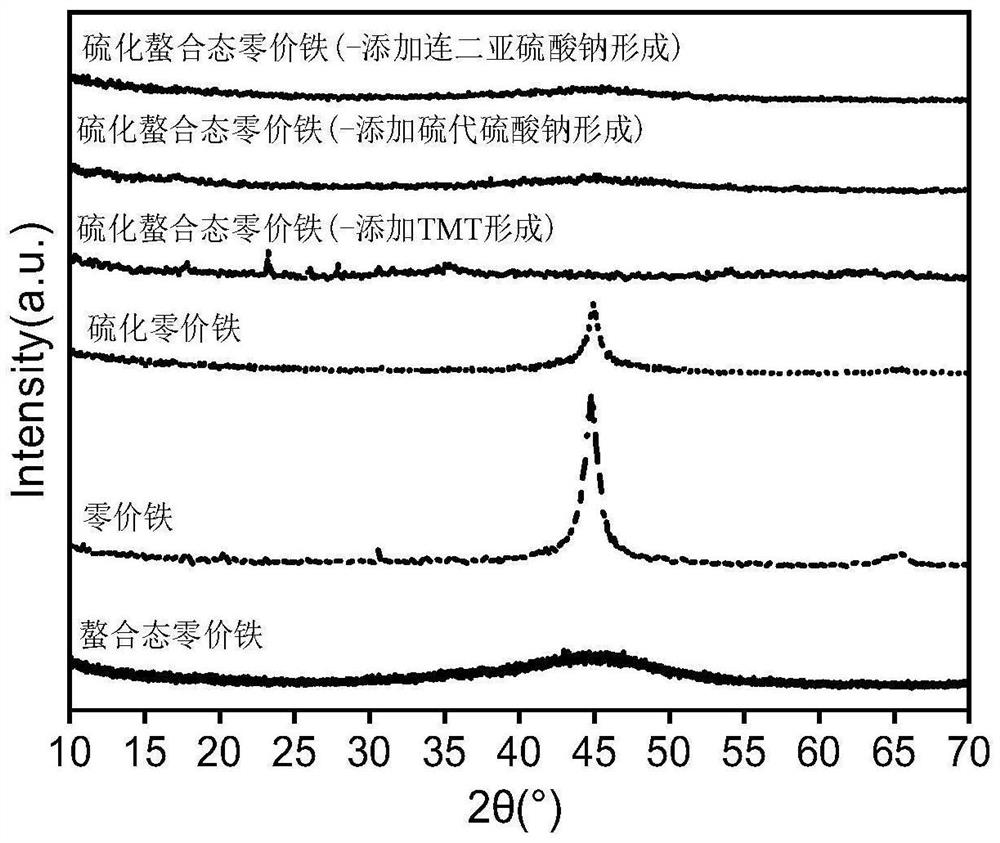
Vulcanized chelated zero-valent iron as well as preparation method and application thereof
A technology of zero-valent iron and chelated state, which is applied in chemical instruments and methods, water/sludge/sewage treatment, water/sewage treatment, etc., can solve the problems of easy aggregation of zero-valent iron and high resistance of iron oxide film, and achieve Good adsorption performance, resistance reduction, and the effect of overcoming defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preparation method of sulfurized chelated zero-valent iron, comprising the following steps:
[0041] Add 3.5000g of ferrous sulfate and 4mL of N,N-dimethylformamide into a three-necked flask filled with 200mL of ultrapure water at normal temperature and pressure, and stir for 5 minutes to obtain a mixed solution; In a 100mL beaker, add 4.0000g sodium borohydride, 75mL ultrapure water, stir and dissolve to obtain NaBH 4 solution; at normal temperature and pressure, the NaBH 4 The solution was added dropwise to the mixed solution, the solution turned black and gradually formed particles, NaBH 4 After the dropwise addition of the solution was completed, 3.5000 g of triazine trithiol trisodium salt hydrate (TMT) was added, stirred and reacted for 15 minutes, separated and washed, and freeze-dried at -60° C. under vacuum to obtain sulfide-chelated zero-valent iron.
Embodiment 2
[0043] A preparation method of sulfurized chelated zero-valent iron, comprising the following steps:
[0044] Add 4.0000g of ferrous sulfate and 5mL of N,N-dimethylformamide into a three-necked flask filled with 250mL of ultrapure water at normal temperature and pressure, and stir for 10 minutes to obtain a mixed solution; In a 100mL beaker, add 4.7000g sodium borohydride, 90mL ultrapure water, stir and dissolve to obtain NaBH 4 solution; at normal temperature and pressure, the NaBH 4 The solution was added dropwise to the mixed solution, the solution turned black and gradually formed particles, NaBH 4 After the dropwise addition of the solution was completed, 3.7000 g of sodium thiosulfate was added, stirred and reacted for 17 minutes, separated and washed, and freeze-dried at -55° C. under vacuum to obtain zero-valent iron in a sulfide chelated state.
Embodiment 3
[0046] A preparation method of sulfurized chelated zero-valent iron, comprising the following steps:
[0047] Add 5.2000g of ferrous sulfate and 6mL of N,N-dimethylformamide into a three-necked flask filled with 300mL of ultrapure water at normal temperature and pressure, and stir for 15 minutes to obtain a mixed solution; In a 100mL beaker, add 5.0000g sodium borohydride, 100mL ultrapure water, stir and dissolve to obtain NaBH 4 solution; at normal temperature and pressure, the NaBH 4 The solution was added dropwise to the mixed solution, the solution turned black and gradually formed particles, NaBH 4 After the solution was added dropwise, 4.0000 g of sodium dithionite was added, stirred and reacted for 20 minutes, separated and washed, and freeze-dried at -50° C. under vacuum to obtain sulfide-chelated zero-valent iron.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com