Dendritic cell vaccine sensitized by A2B5 + glioma cells
A technology of dendritic cells and glioma stem cells, applied in the fields of biotechnology and medicine, can solve the problem of limited ability of sensitizing T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277] Example 1. Preparation of A2B5+ glioma stem cell antigen
[0278] Take out the fresh human glioma tissue block and wash it 2-3 times with PBS containing double antibody, cut into 1mm 3 size. Wash 2-3 times with DMEM / F12 culture medium, add 5-10 times the tissue volume of type IV collagenase, shake and digest at 200rpm in a 37°C incubator for 15min, centrifuge at 1000rpm / min for 5min, filter with a 100μm cell sieve, and prepare a single cell suspension.
[0279] Add 5 mL of erythrocyte lysate to the cell pellet of the above single-cell suspension, incubate in a 37°C incubator for 5-10 min, wash with PBS three times; use serum-free culture medium (containing 20 ng / mL bFGF, 20 ng / mL EGF and DMEM / F12 culture medium of B27) to resuspend the cell pellet, count and press 2~4×10 5 Cells / mL were inoculated in an ultra-low adsorption six-well plate.
[0280] The cell pellet was collected, and A2B5+ cells were sorted by magnetic beads. Gamma ray irradiation, the irradiated ...
Embodiment 2
[0281] Example 2. Transfection, maturation induction and specificity of DC CTL preparation of
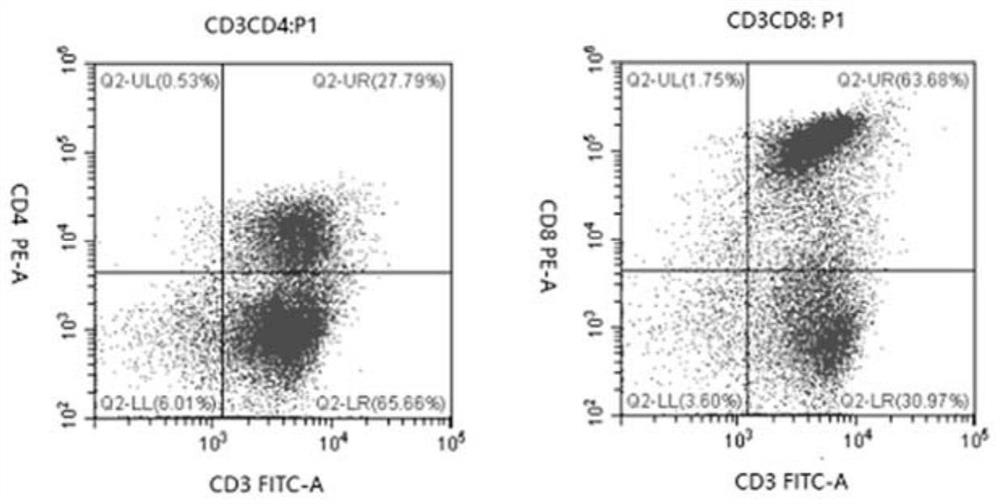
[0282] 1) DC transfection and maturation induction: the patient's own peripheral blood was collected, and autologous plasma (inactivated at 56°C for 30 minutes, stored at 4°C) and peripheral blood were separated by Ficoll-Hypaque (polysucrose-diatrizoate) density gradient separation method Mononuclear cell PBMC, this PBMC was treated with serum-free x-vivo15 at 5-7×10 7 The amount of cells was plated in a T75 culture flask, and the suspension cells were collected after 1 hour for the separation of CD3+ T lymphocytes, and the adherent cells were replaced with IL-4 (100ng / mL), GM-CSF (100ng / mL) and autologous plasma (2 %) of 1640 medium stimulation induced monocytes to differentiate into DC.
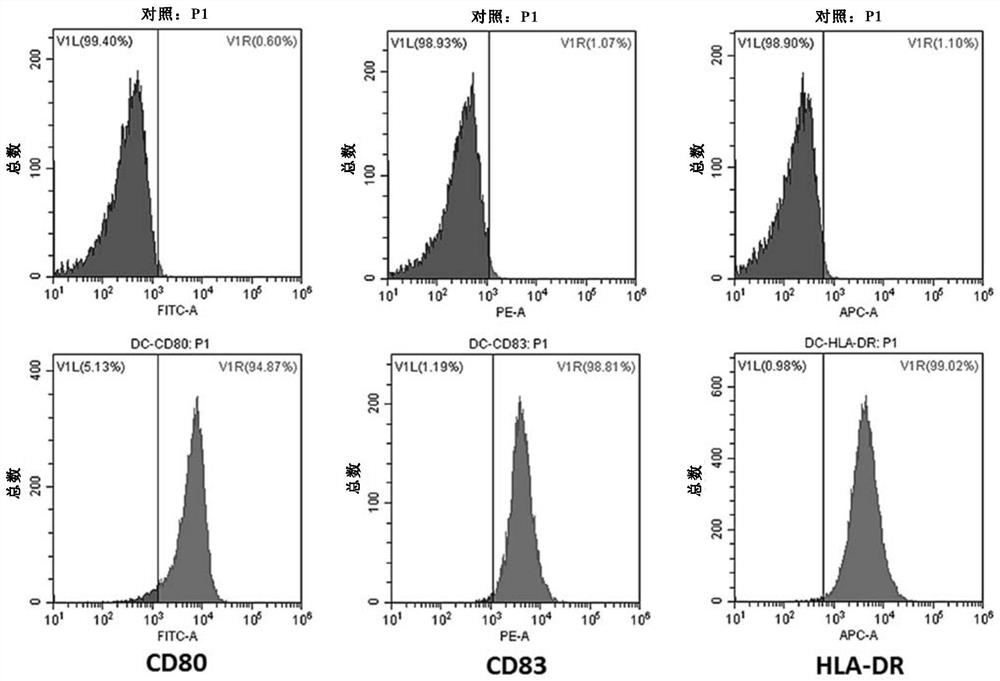
[0283] On day 5, immature DCs suspended in a 24-well plate were sensitized with the A2B5+ stem cell antigen prepared as in Example 1. After 24 hours of sensitization, replace with fresh DC...
Embodiment 3
[0288] Example 3. Antigen-specific CTL tumor cell killing experiment
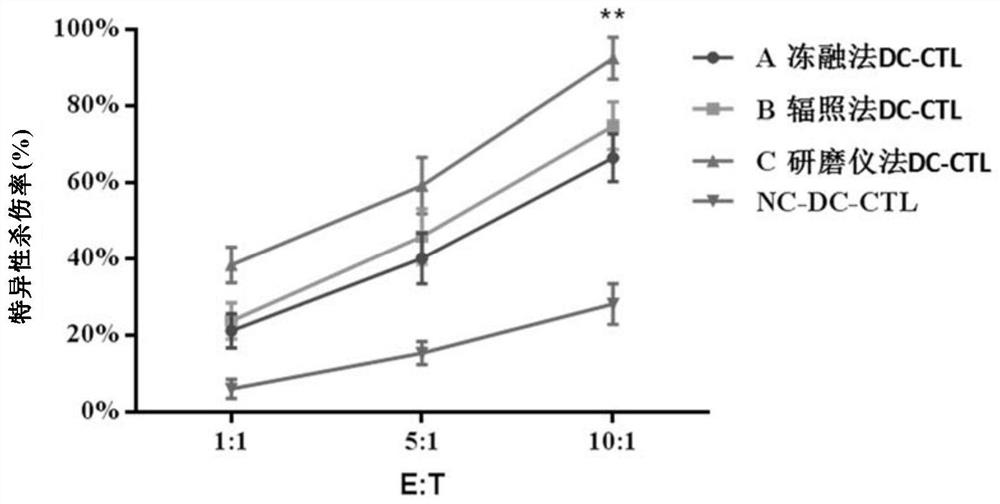
[0289] In order to verify the specific killing effect of antigen-specific CTLs on glioma stem cells, the target cells of this example were sorted A2B5+ cells, and an in vitro killing verification experiment of antigen-specific CTLs was carried out. On the 14th day of specific expansion of T lymphocytes, CTL and tumor target cells were added to 96-well plates at 1:1, 5:1 and 10:1, respectively, and incubated for 16 hours, then added CCK8 kit reagents and incubated for 1 hour. The OD value of cells in each group was detected by a microplate reader, and finally the specific killing efficiency of CTL on cell lines was calculated.
[0290] Such as image 3 The results shown show that the A2B5-targeting CTL prepared in Example 2 has a good killing effect and strong specificity on A2B5+ cells, and the tumor killing rate of conventional CTL is 30% of the tumor killing rate of the present invention. -60%. This ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com