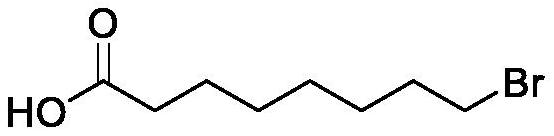
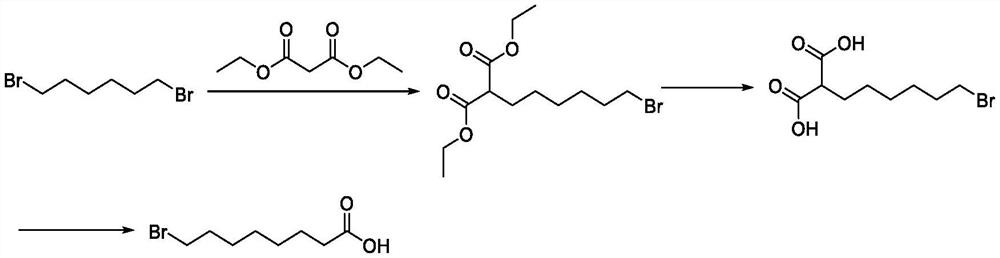
Preparation method of halogenated acid compound
A compound and acid technology, which is applied in the field of preparation of halogenated acid compounds, can solve the problems of poor safety and controllability, severe reaction triggering, increased pressure on wastewater treatment, etc., and achieves the effects of low toxicity, high yield, toxicity and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Dissolve 5.9 g of sodium chlorite (52.19 mmol) with a mass fraction of 80% in 18 g of water and set aside.
[0080] Add 8-bromooctyl alcohol 10g (47.82mmol), acetonitrile 39g, water 18g, TEMPO 0.07g (0.45mmol) in the reaction bottle, warm up to 30 ℃, dropwise add 6% sodium hypochlorite aqueous solution 0.5g (0.40mmol), dropwise The sodium chlorite aqueous solution 4.8g (10.48mmol) that has configured, drips and feeds carbon dioxide gas to keep the reaction system at pH=6~7 all the time, after the reaction triggers, keep internal temperature 30 ℃, continue to dropwise add 1.25g ( 1.0mmol) 6% sodium hypochlorite aqueous solution and 19.1g (41.71mmol) of the remaining sodium chlorite aqueous solution prepared. After dropping, the reaction was stirred at 30°C for 2h. Control the temperature below 30°C, add dropwise 20% sodium sulfite aqueous solution to quench the reaction, add 33% dilute sulfuric acid to adjust pH = 3, stand to separate layers, and concentrate the organic...
Embodiment 2
[0083] Dissolve 12g of sodium chlorite (106.15mmol) with a mass fraction of 80% in 40g of water for use.
[0084] Add 20g (95.64mmol) of 8-bromooctyl alcohol, 80g of acetonitrile, 40g of water, 0.15g (0.96mmol) of TEMPO into the reaction flask, raise the temperature to 35°C, add 1g (0.81mmol) of 6% sodium hypochlorite aqueous solution dropwise, and add dropwise to configure 10g (20.41mmol) of a good sodium chlorite aqueous solution, after the dropwise addition of carbon dioxide gas to keep the reaction system at pH = 5 ~ 6, after the reaction is triggered, keep the internal temperature at 35 ° C, continue to drop 2.5g (2.02mmol ) 6% sodium hypochlorite aqueous solution and the remaining sodium chlorite aqueous solution 42g (85.74mmol) configured. After dropping, the reaction was stirred at 35°C for 1h. Control the temperature below 35°C, add 20% sodium sulfite aqueous solution dropwise to quench the reaction, add 33% dilute sulfuric acid to adjust pH = 2, let stand to separat...
Embodiment 3
[0086] Dissolve 1.2 kg of sodium chlorite (10.61 mol) with a mass fraction of 80% in 4 kg of water and set aside.
[0087] 2kg (9.6mol) of 8-bromooctyl alcohol, 8kg of acetonitrile, 4kg of water, TEMPO14.7g (0.094mol) were added to the 50L reactor, and the temperature was raised to 20°C, and 100g (0.08mol) of 6% sodium hypochlorite aqueous solution was added dropwise. 1 kg (2.04 mol) of the prepared sodium chlorite aqueous solution, after the dropwise feeding of carbon dioxide gas to keep the reaction system at pH = 5 ~ 6, after the reaction is triggered, keep the internal temperature at 20 ° C, continue to drop 250 g (0.20 mol ) 6% sodium hypochlorite aqueous solution and 4.2kg (8.57mol) of the remaining sodium chlorite aqueous solution configured. After dropping, the reaction was stirred at 20°C for 1.5h. Control the temperature below 20°C, add 20% sodium sulfite aqueous solution dropwise to quench the reaction, add 33% dilute sulfuric acid to adjust pH = 3, stand to separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com