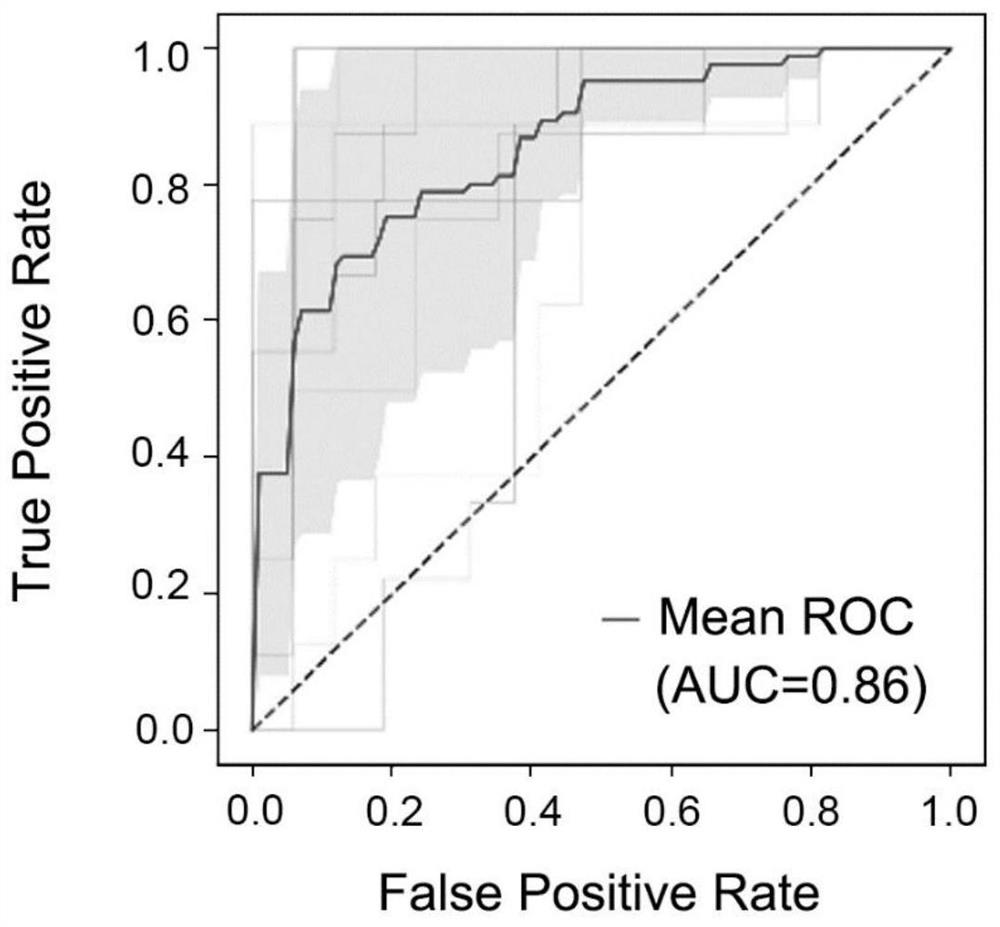
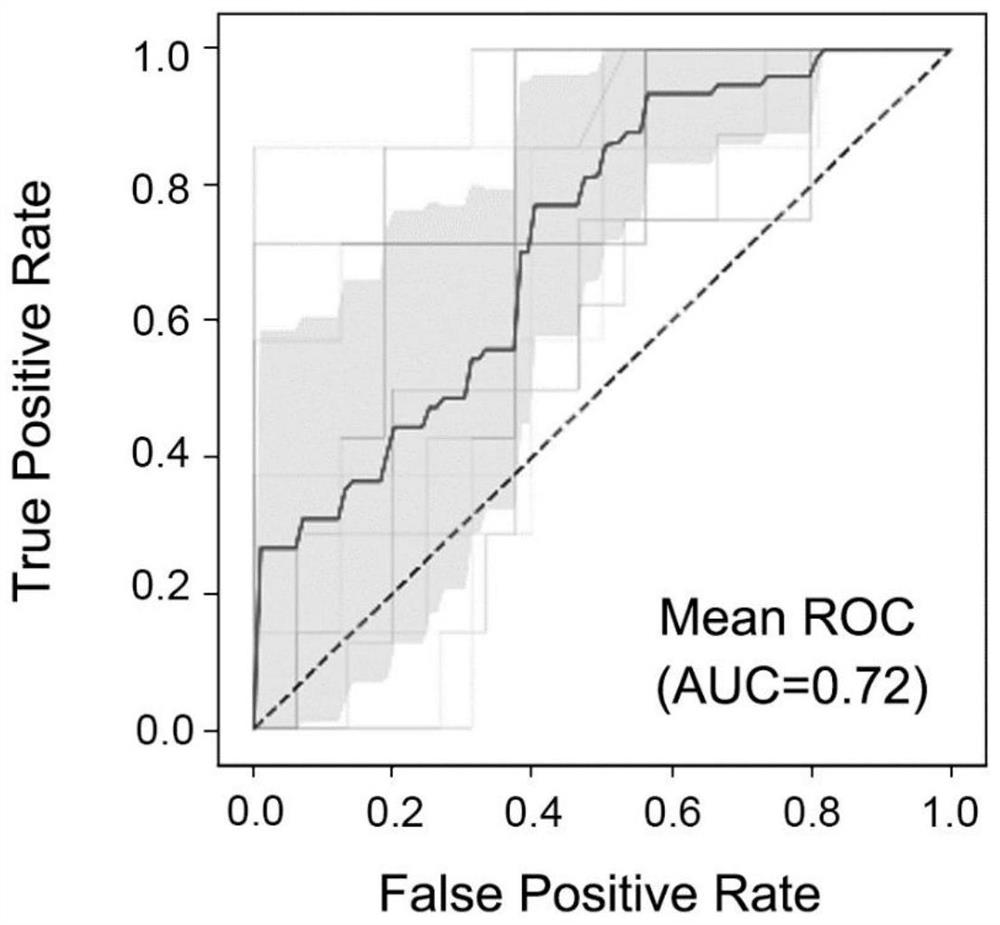
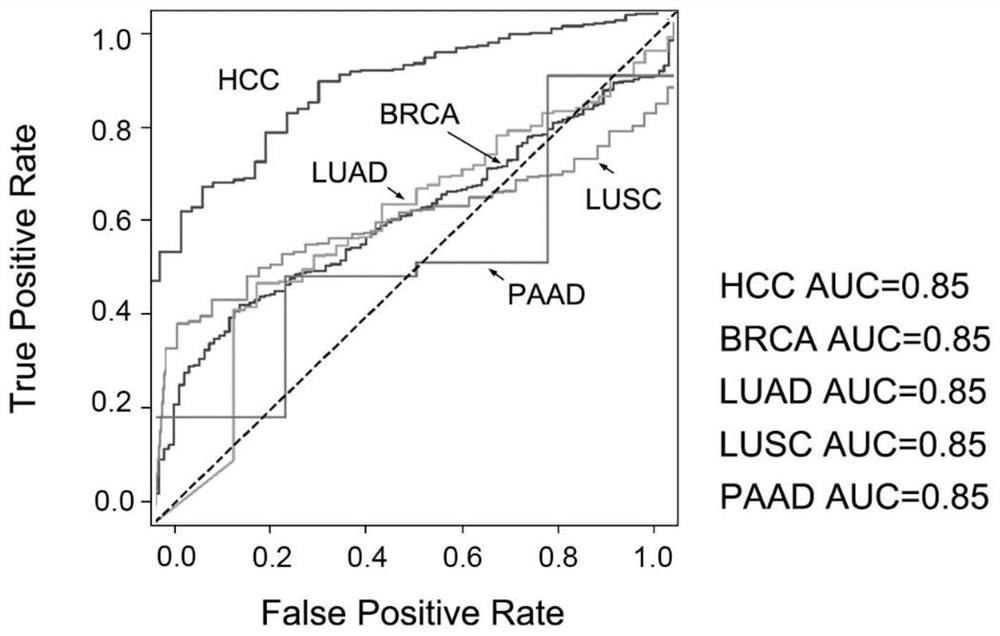
Application of miRNA combined marker in preparation of kit for diagnosing and detecting early liver cancer
A technology for early detection of liver cancer, applied in the fields of molecular biology and medical diagnosis, to achieve the effect of accurate results, strong specificity, and reduced errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Subject and sample extraction
[0022] The samples were provided by Singapore General Hospital (approved by Singapore SingHealth CIRB B, approval number CIRB Ref: 2007 / 447 / B). Among them, there were 86 cases of HCC patients, 154 cases of non-HCC liver disease patients, and 12 cases of healthy people. Among non-HCC liver disease patients, there were 59 HBV-positive cirrhotic patients, 89 HBV-positive non-cirrhotic patients, and 6 HBV-negative cirrhotic patients.
Embodiment 2
[0023] Example 2 Extraction of exosomes
[0024] Collect 2ml of peripheral blood from the subject with a disposable syringe, quickly transfer it to an EDTA anticoagulant tube, and mix well. Separate the plasma within 2 hours of collection. The specific steps are: 1) transfer the whole blood to a 2ml centrifuge tube, and centrifuge at 500g for 10min at room temperature; 2) take the upper plasma, and centrifuge at 2000g for 10min at 4°C; 3) take the upper plasma, 4°C, Centrifuge at 16,000 g for 10 min; 4) The separated plasma is directly used for exosome extraction or frozen in a -80°C refrigerator.
[0025] Exosomes were extracted according to the instructions of the exosome extraction kit Exo-Quick exosome precipitation solution (EXOQ5SA-1, SBI, United States). 1) Add the plasma to a centrifuge tube, and add Exo-Quick at a ratio of 63 μl for every 250 μl of plasma; 2) Centrifuge at 1500 g at 4°C for 30 minutes, discard the supernatant; 3) Centrifuge at 1500 g at 4°C for 5 min...
Embodiment 3
[0026] Example 3 Extraction of exosome miRNA
[0027] miRNA was extracted according to the operating instructions of the miRNeasy Serum / PlasmaKit kit (217184, QIAGEN, Germany). The specific steps are: 1) Add 5 times the volume of QIAzol LysisReagent to the plasma separated in Example 2, vortex or pipette to mix; 2) Incubate at room temperature for 5 minutes; 3) Add an equal volume of chloroform and shake for 15 seconds; 4) Incubate at room temperature for 2-3min; 5) Centrifuge at 12000g for 15min at 4°C; 6) Transfer the upper layer to a new centrifuge tube, add 1.5 times the volume of absolute ethanol, and mix by pipetting to obtain the sample; 7) Take 700μl sample to RNeasy MinElute Spin column (placed in a 2ml collection tube), cover the lid, centrifuge at room temperature>8000g for 15s, discard; 8) repeat step 7 once; 9) add 700μl RWTbuf to the RNeasy MinElute spin column, centrifuge at room temperature>8000g for 15s, discard ;10) Add 500μl RPE buf to the RNeasy MinElute s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com