Technological method and composition for improving flowability of piperacipril isethionate
A technology of piperidine isethionate and piperidine isethionate, applied in the field of medicine, can solve problems such as poor fluidity, difficulty in large-scale manufacturing, etc., and achieve the effect of good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
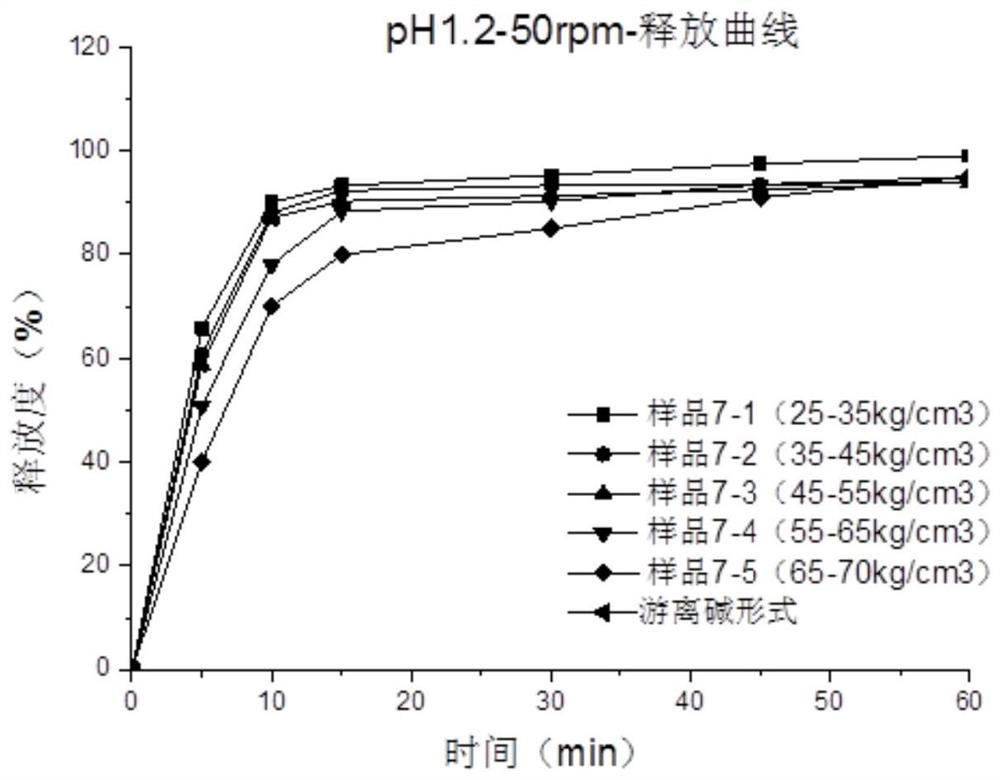
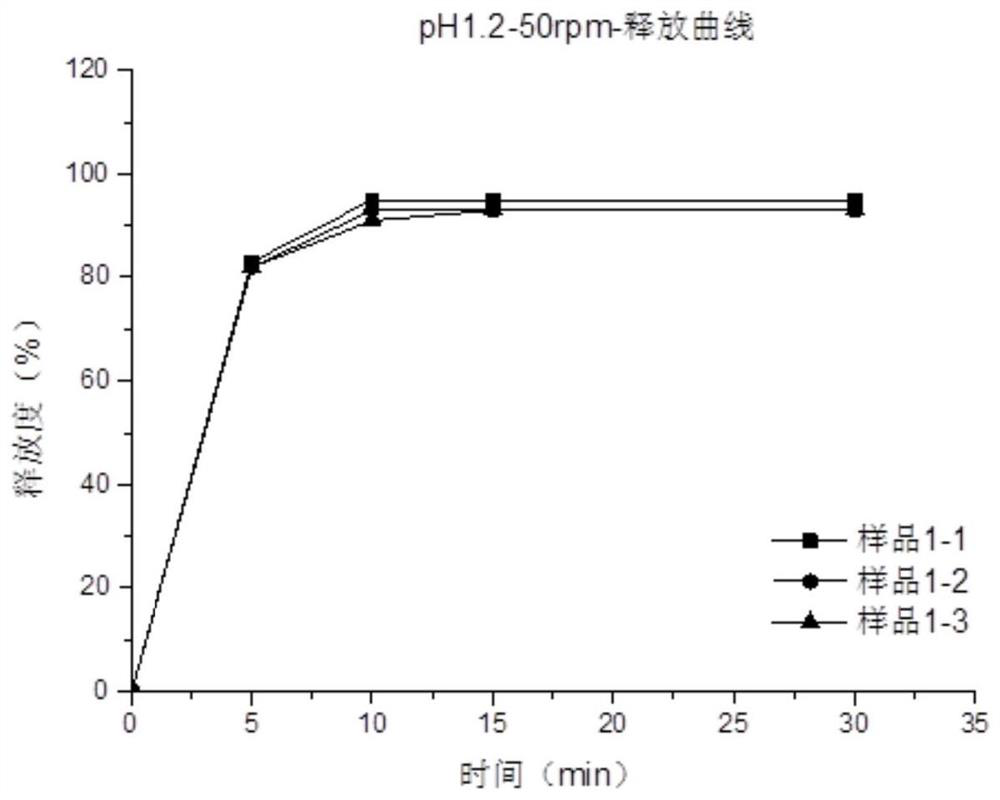
Image
Examples
Embodiment 1
[0050] The composition of the prescription is as follows:
[0051]
[0052]
[0053] Preparation Process:
[0054] 1. Preparation of raw and auxiliary materials
[0055] Palbociclib isethionate, microcrystalline cellulose, and crospovidone are passed through a 40-mesh sieve, and lactose, colloidal silicon dioxide, and magnesium stearate are passed through a 80-mesh sieve for later use.
[0056] 2. Premix
[0057] Weigh respectively palbociclib isethionate and microcrystalline cellulose premixed according to the prescription quantity, pass through a 40-mesh sieve,
[0058] 3. mix
[0059] Add the premix, lactose, crospovidone, and colloidal silicon dioxide into a three-dimensional motion mixer and mix for 15 minutes;
[0060] 4. Dry granulation
[0061] After setting the parameters of the dry granulator (roller pressure is set to 35-45kg / cm 3 ), the mixture obtained in step 3 is subjected to dry granulation;
[0062] 5. Total mix
[0063] Add the magnesium stearat...
Embodiment 2
[0084] The composition of the prescription is as follows:
[0085]
[0086] 1. Preparation of raw and auxiliary materials
[0087] Palbociclib isethionate, pregelatinized starch, and crospovidone are passed through a 40-mesh sieve, and lactose, colloidal silicon dioxide, and magnesium stearate are passed through a 80-mesh sieve for later use.
[0088] 2. Premix
[0089] Weigh and premix palbociclib isethionate and pregelatinized starch respectively according to the prescription quantity, pass through a 40-mesh sieve,
[0090] 3. mix
[0091] Add the premix, lactose, crospovidone, and colloidal silicon dioxide into a three-dimensional motion mixer and mix for 15 minutes;
[0092] 4. Dry granulation
[0093] After setting the parameters of the dry granulator (roller pressure is set to 35-45kg / cm 3 ), the mixture obtained in step 3 is subjected to dry granulation;
[0094] 5. Total mix
[0095] Add the magnesium stearate and the granules obtained after dry granulation i...
Embodiment 3
[0099] The composition of the prescription is as follows:
[0100]
[0101] Preparation Process:
[0102] 1. Preparation of raw and auxiliary materials
[0103]Pass through a 40-mesh sieve, palbociclib isethionate, anhydrous calcium hydrogen phosphate, and crospovidone, and pass through a 80-mesh sieve for talcum powder and sodium stearyl fumarate for later use.
[0104] 2. Premix
[0105] Weigh respectively palbociclib isethionate and anhydrous calcium hydrogen phosphate premixed according to the prescription quantity, pass through a 40-mesh sieve,
[0106] 3. mix
[0107] Add the premix, talcum powder and crospovidone into the three-dimensional motion mixer and mix for 15 minutes;
[0108] 4. Dry granulation
[0109] After setting the parameters of the dry granulator (roller pressure is set to 35-45kg / cm 3 ), the mixture obtained in step 3 is subjected to dry granulation;
[0110] 5. Total mix
[0111] Add sodium stearyl fumarate and the granules obtained by dry g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com