Albumin HSA-Hydrophobic-IIB with self-assembly performance and application thereof
A technology of albumin and recombinant bacteria, applied in the field of biomedicine, can solve the problems of limited drug application range, low solubility, difficult absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1. HSA-Hydrophobic-IIB gene design and protein expression
[0033] 1. Genetic design
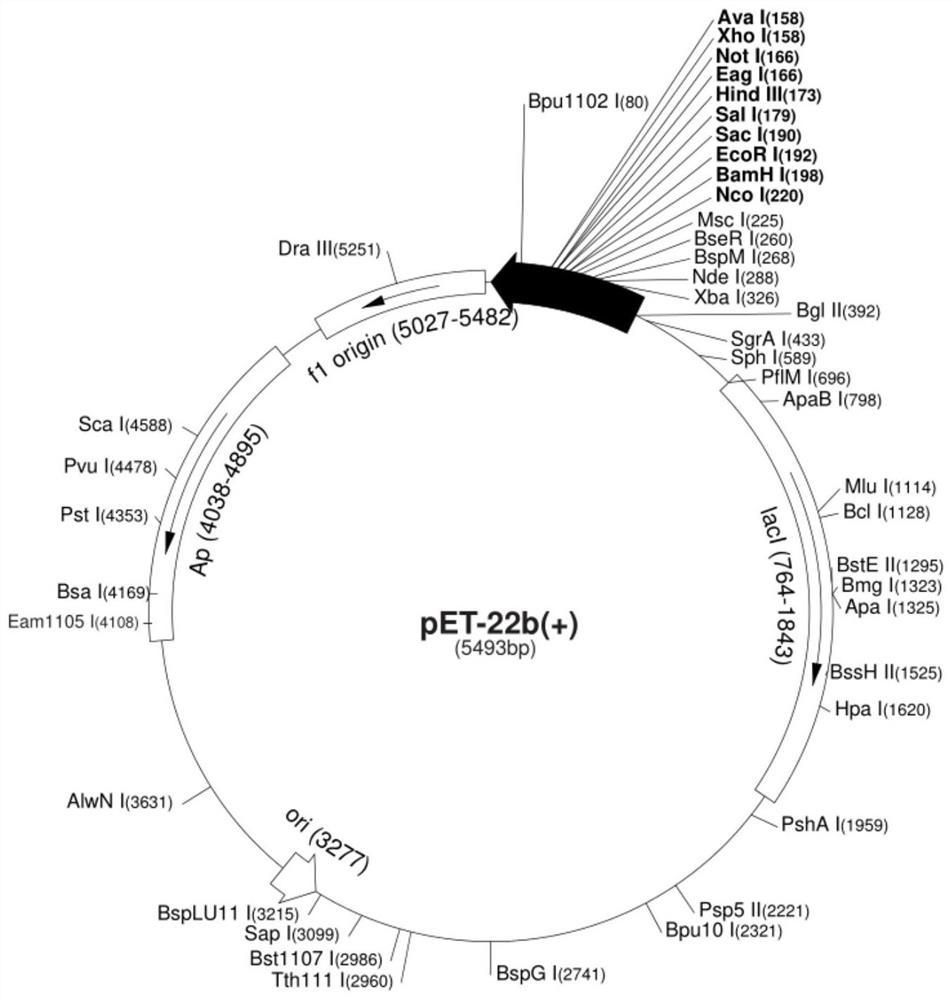
[0034] The inventor designed a gene, the nucleotide sequence of which is shown in SEQ ID No.2, with a full length of 1779bp. The gene encodes a protein whose amino acid sequence is shown in SEQ ID No.1, with a total length of 592 amino acids, named HSA-Hydrophobic-IIB novel albumin. Sangon Bioengineering (Shanghai) Co., Ltd. was entrusted to carry out the whole gene synthesis of the gene and added NdeI restriction site (CATATG) and XhoI restriction site (CTCGAG) to the 5' end and 3' end of the gene respectively. The gene sequence with restriction sites is shown in SEQ ID No.3. The synthesized gene was sequenced and verified, and the gene with the correct sequence was used for subsequent vector construction.
[0035] Amino acid sequence of HSA-Hydrophobic-IIB (592aa)
[0036]MDAHKSEVAHRFKDLGEENFKALVLIAFAQYLQQCPFEDHVKLVNEVTEFAKTCVADESAENCDKSLHTLFGDKLCTVATLRETYGEMADCCAKQEPERN...
Embodiment 2
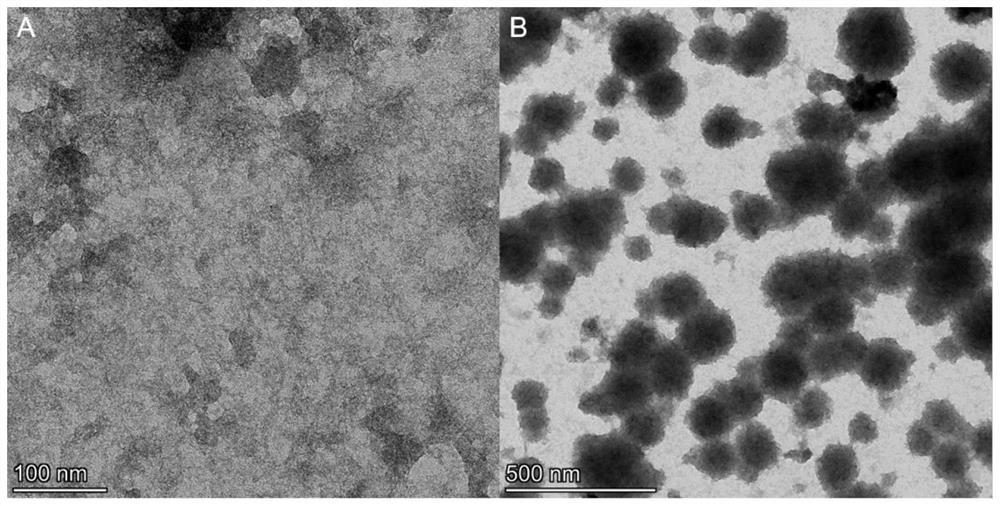
[0060] Example 2. Preparation of Nanomicelles of Natural Human Serum Albumin (ori-HSA)
[0061] The amino acid sequence of native human serum albumin (ori-HSA) is shown in SEQ ID No.4, with a total length of 591 amino acids; NdeI enzyme cleavage sites (CATATG ) and XhoI restriction site (CTCGAG), to obtain the gene whose nucleotide sequence is shown in SEQ ID No.5, the full length is 1788bp. Sangon Bioengineering (Shanghai) Co., Ltd. was commissioned to synthesize the gene, and the synthesized gene was sequenced and verified, and the gene with the correct sequence was used for subsequent vector construction.
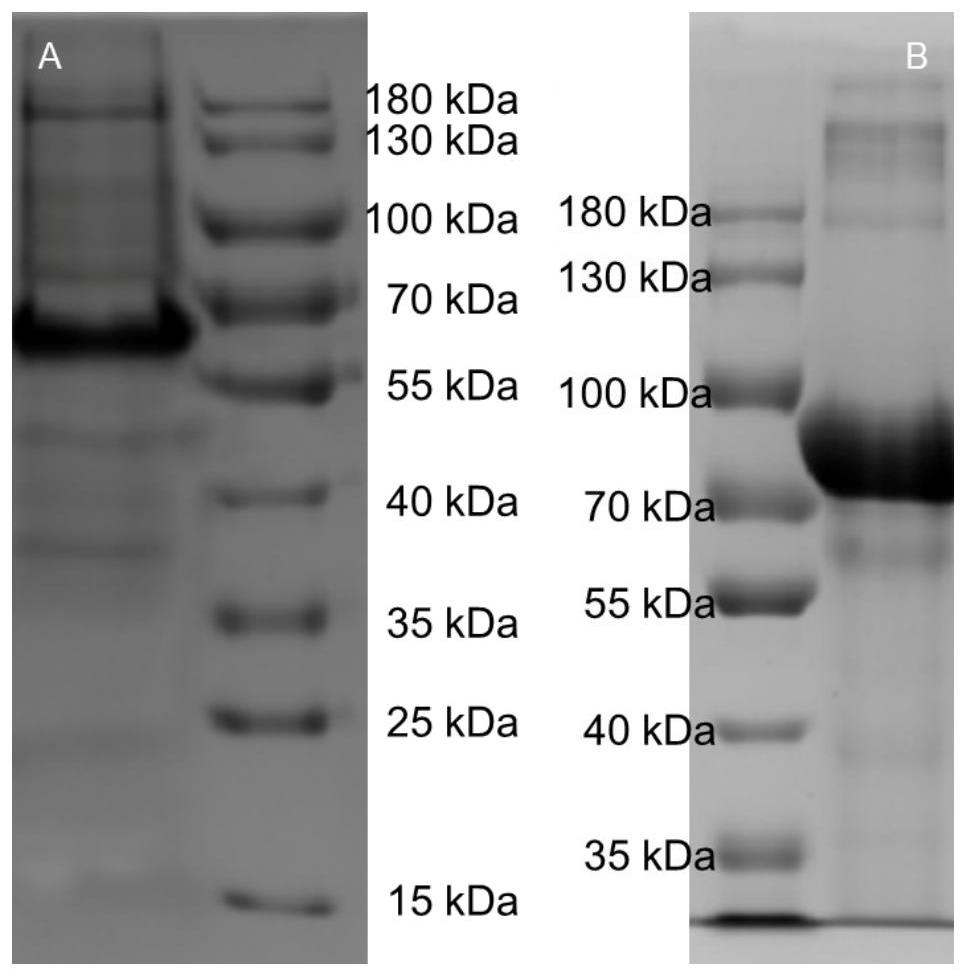
[0062] The methods of prokaryotic expression vector construction, transformation, protein expression and purification of native human serum albumin (ori-HSA) are consistent with the methods of prokaryotic expression vector construction, transformation, protein expression and purification of HSA-Hydrophobic-IIB novel albumin, detailed The steps are the same as 2-4 in Exa...
Embodiment 3
[0064] Example 3. Cytotoxicity evaluation of HSA-Hydrophobic-IIB novel albumin
[0065] The toxicity of HSA-Hydrophobic-IIB new albumin to human embryonic kidney cell 293T (Cell Bank of Type Culture Collection Committee, Chinese Academy of Sciences) was studied by CCK8 method to evaluate the biological safety of the new albumin HSA-Hydrophobic-IIB. In a 96-well plate, inoculate 1×10 per well 4 Human embryonic kidney cells 293T were placed in DMEM medium (gibco), and 5 experimental groups and 1 control group were set up, and 3 replicate wells were set up for each group. After culturing the cells at 37°C for 24 hours, add HSA-Hydrophobic-IIB new albumin to the wells in the experimental group (experimental wells), so that the final concentrations of HSA-Hydrophobic-IIB new albumin in the five experimental groups were 10 μg / ml , 50 μg / ml, 100 μg / ml, 200 μg / ml, 400 μg / ml. In the wells of the control group (control wells), HSA-Hydrophobic-IIB novel albumin was not added. Continue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com