PCR (Polymerase Chain Reaction) primer group and kit for jointly detecting various respiratory viruses
A detection primer and joint detection technology, applied in the biological field, can solve the problems of multiple detection links, complex and cumbersome operations, and low throughput, and achieve the effects of shortened detection time, accurate detection, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
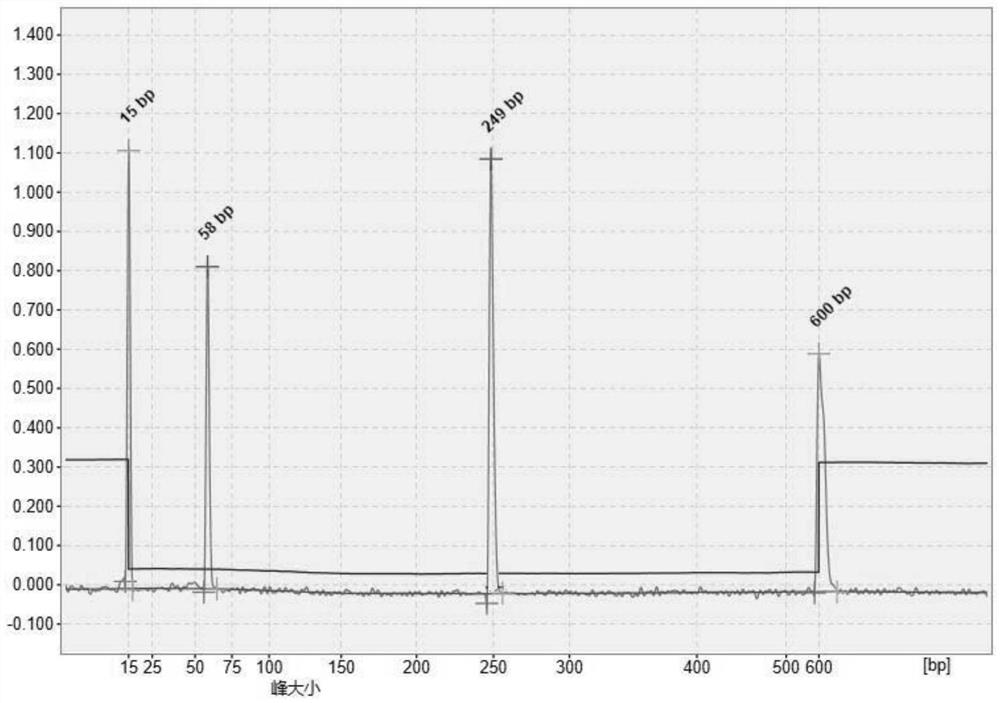
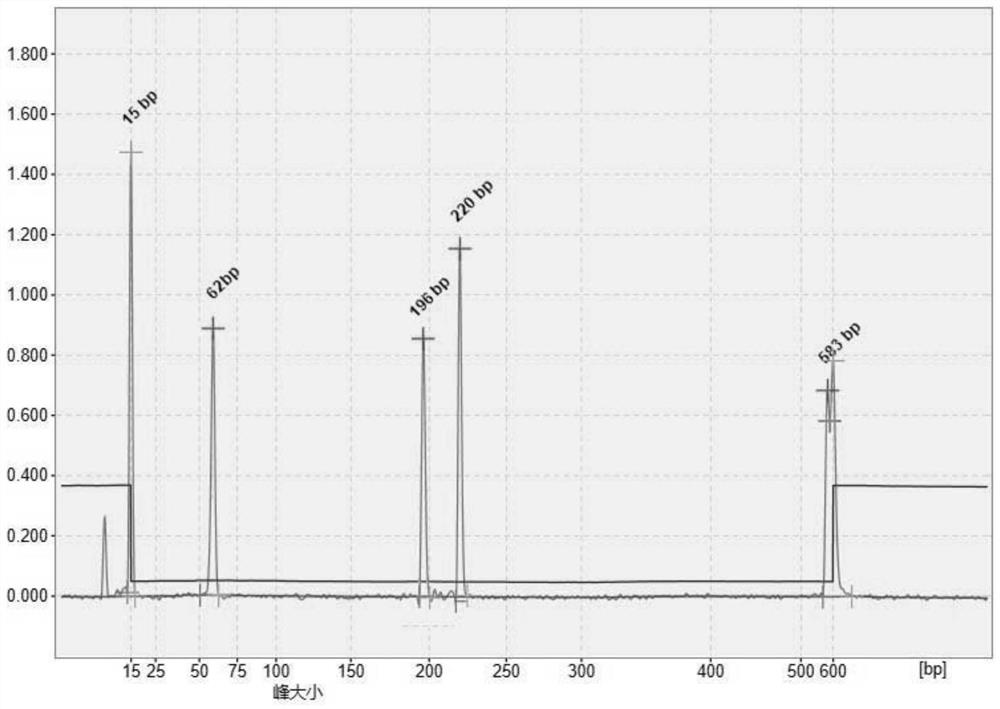
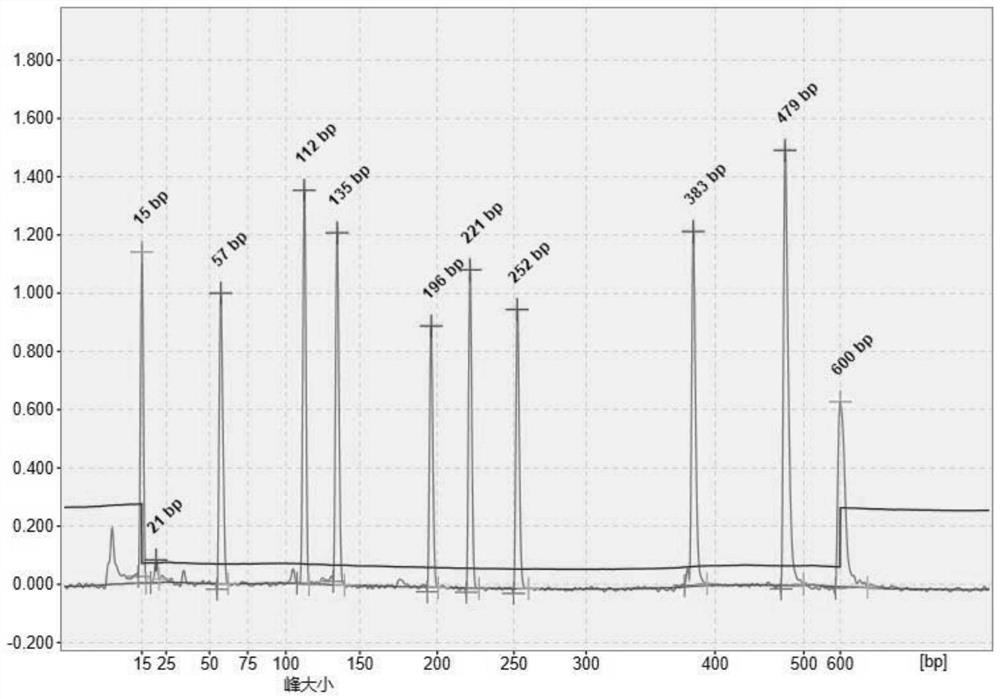
Image
Examples
Embodiment 1
[0063] When the examples give numerical ranges, it should be understood that, unless otherwise stated in the present invention, the two endpoints of each numerical range and any value between the two endpoints can be selected. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art. In addition to the specific methods, equipment, and materials used in the embodiments, according to those skilled in the art's grasp of the prior art and the description of the present invention, the methods, equipment, and materials described in the embodiments of the present invention can also be used Any methods, devices and materials of the prior art similar or equivalent to the practice of the present invention. The preparation and use method of embodiment 1 kit
[0064] The primers for detecting influenza A virus, the primers for influenza A virus H1N1 (2009), the primers for influenza A virus H...
Embodiment 2
[0077] The detection effect evaluation of embodiment 2 kit
[0078] The embodiment of the present invention provides a specific usage method of the kit. Each pair of detection primers shown in the sequence in Table 1, the pair of exogenous RNA internal reference amplification primers, and the OneStep RT-PCR Enzyme MIX purchased from QIAGEN Mix evenly to obtain multiple RT-PCR reactions, and the concentration of each primer in the multiple PCR reactions is shown in Table 3.
[0079] table 3
[0080]
[0081]
[0082] 1. Collect nasopharyngeal swab specimens from patients infected with human respiratory syncytial virus or influenza A virus H1N1 (2009) according to the standard operation. After collection, they should be placed in ice packs and sent for inspection immediately.
[0083] 2. After the sample is pre-treated, 10 μL of exogenous RNA internal reference is added to jointly extract the RNA in the sample to obtain the RNA sample to be tested.
[0084] 3. Preparation ...
Embodiment 3
[0099] Sensitivity and specificity analysis of embodiment 3 kit
[0100] sensitivity analysis:
[0101] Perform 10-fold serial dilutions on the positive quality control products of the seven virus detection targets, and the concentrations are 1×10 6 copies / mL, 1×10 5 copies / mL, 1×10 4 copies / mL, 1×10 3 copies / mL, 1×10 2 copies / mL and 10copies / mL, repeat 3-5 samples for each gradient dilution, use the same as in Example 2, determine the seven virus multiple PCR detection system, carry out multiple PCR amplification and fragment analysis detection, until If no signal was detected, 20 repeated tests were performed on each copy, and the positive detection rate level of 95% was used as the lowest lower limit of detection (LOD), which was the sensitivity.
[0102] The detection sensitivity of kit of the present invention, as shown in the following table:
[0103] Table 6
[0104]
[0105]
[0106] Specific analysis:
[0107] The specificity of the detection method esta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com