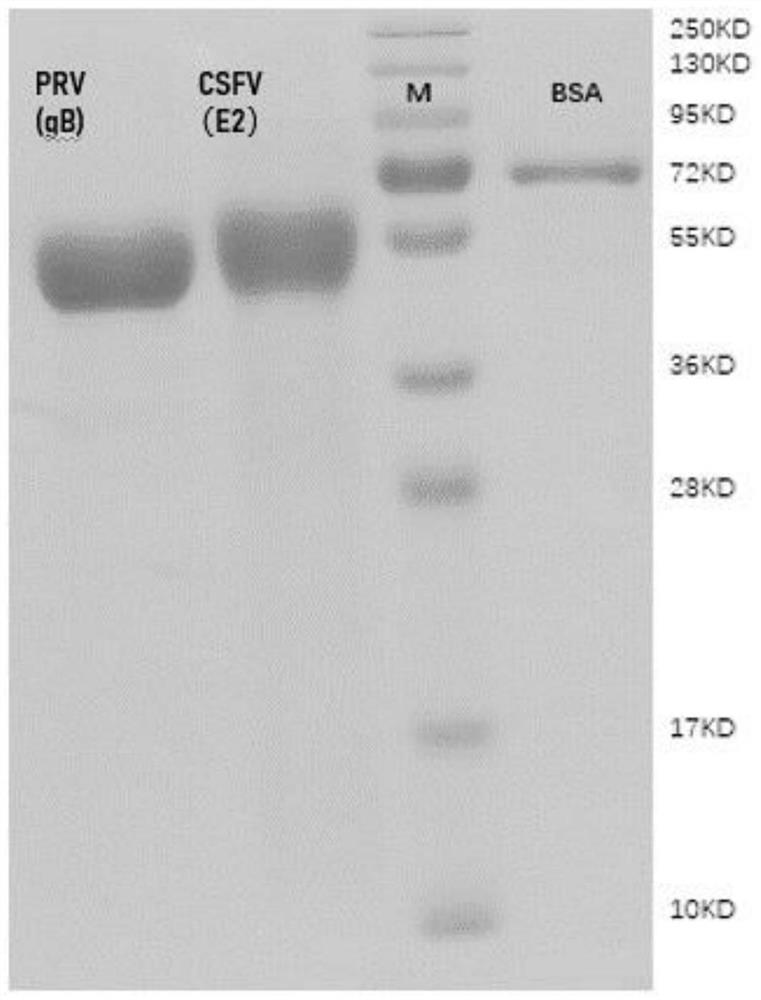
Chog cholera and porcine pseudorabies bivalent vaccine as well as preparation method and application thereof
A technology of swine pseudorabies and dual vaccine, which is applied in the field of swine vaccine, can solve the problems of swine fever infection and the difficulty of swine fever vaccine to play an epidemic prevention role, and achieve the effect of improving protection efficiency and speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] vaccine preparation
[0076] (1) Preparation of target gene
[0077] Design primer, amplify classical swine fever virus and porcine pseudorabies virus antigenic protein gene by the method for PCR; The amplification primer of the antigenic protein gene designed in this process one is:
[0078] CFSV E2 upstream primer: 5'-TAGGCTTCTATTCTAGTCTGCGCAATA-3' (contains XbaI restriction site)
[0079] CFSV E2 downstream primer: 5'-GAATGAAGATTATGCTATGGTACGTAG-3' (contains KpnI restriction site)
[0080] PRV gB upstream primer is: 5'-CCAGTCGCAGGCCACCGTGAGTG-3' (contains KpnI restriction site)
[0081] The downstream primer of PRV gB is: 5'-ATCGCGTGCTCTTCATGGAGATC-3' (XbaI restriction site)
[0082] Among them, the amplified length of CSFV E2 gene is 294bp, and the amplified length of PRV gB gene is 632bp.
[0083] (2) Construction of recombinant replication-deficient chimpanzee adenovirus shuttle vector
[0084] Insert the antigenic protein antigenic protein gene of the classi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com