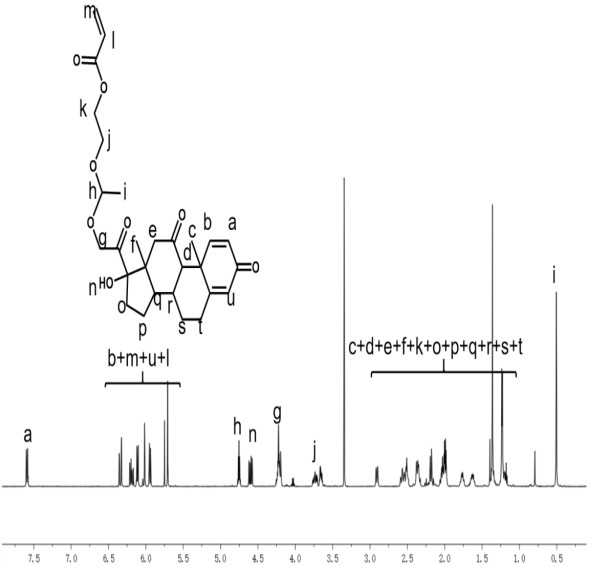
Acid-responsive hyperbranched poly-prodrug nano-micelle as well as preparation method and application thereof
A hyperbranched polymer and nanomicelle technology, which is applied in the field of biomedical materials, can solve problems such as increased blood circulation time, and achieve the effects of low cytotoxicity, improved bioavailability and stability, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of an acid-responsive hyperbranched polyprodrug nanomicelle
[0055] The hydrophobic drug is prednisone (PNS), the compound 1 is VEA, the RAFT chain transfer agent is MTDDA (4-methyl-9-thioxo-3,5-dioxa-8,10-dithiatridecy acrylate), the water-soluble monomer D is OEGMA and the catalyst is azobisisobutyronitrile (AIBN).
[0056] 1. Synthesis of PNS-acetal-methacrylate
[0057] Prednisone PNS (1mmol) was dissolved in 20mL of anhydrous tetrahydrofuran to prepare solution 1, PPTS (pyridine p-toluenesulfonate 1.3g mmol) was dissolved in 1mLDMSO, and then added to solution 1, in ice bath, N 2 Under protection, 5 mL of anhydrous tetrahydrofuran containing compound 1 (VEA, ethylene-vinyl acetate copolymer) (2 mmol) was added dropwise, and then allowed to rise naturally to room temperature to react overnight. Add 0.5g K 2 CO 3 Terminate the reaction, stir for 30 minutes and filter with a G5 sand core funnel and filter paper. The filtrate was collected,...
Embodiment 2
[0070] Example 2: Preparation of an acid-responsive hyperbranched polyprodrug nanomicelle
[0071] The hydrophobic drug is simvastatin (SIM), the compound 1 is VEA, the RAFT chain transfer agent is MTDDA, the water-soluble monomer D is OEGA and the catalyst is azobisisobutyronitrile (AIBN). Specific steps are as follows:
[0072] 1. Synthesis of SIM-acetal-methacrylate:
[0073] Simvastatin (SIM) (1mmol) was dissolved in 20mL of anhydrous tetrahydrofuran to prepare solution 1, PPTS (pyridine p-toluenesulfonate 1.3g mmol) was dissolved in 1mLDMSO, and then added to solution 1, under ice bath and N2 protection 5 mL of anhydrous tetrahydrofuran containing compound 1 (VEA, ethylene-vinyl acetate copolymer) (2 mmol) was added dropwise, and then allowed to rise to room temperature to react overnight. Add 0.5g K 2 CO 3 Terminate the reaction, stir for 30 minutes and filter with a G5 sand core funnel and filter paper. The filtrate was collected, and the solvent and volatile matte...
Embodiment 3
[0079] Example 3: Preparation of an acid-responsive hyperbranched polyprodrug nanomicelle
[0080] The hydrophobic drug is coupled with epicatechin (EC), compound 1 as VEA, RAFT chain transfer agent as MTDDA, water-soluble monomer D as poly(ethylene glycol) methyl ether methacrylate (PEGMA) and catalyst. Azobisisobutyronitrile (AIBN).
[0081] 1. Synthesis of EC-acetal-methacrylate: Dissolve epicatechin EC (1mmol) in 20mL anhydrous tetrahydrofuran to make solution 1, PPTS (pyridine p-toluenesulfonate 1.3g mmol) is dissolved in 1mLDMSO, After adding solution 1, 5 mL of anhydrous tetrahydrofuran containing compound 1 (VEA, ethylene-vinyl acetate copolymer) (2 mmol) was added dropwise in an ice bath under N2 protection, and then allowed to rise to room temperature to react overnight. Add 0.5g K 2 CO 3 Terminate the reaction, stir for 30 minutes and filter with a G5 sand core funnel and filter paper. The filtrate was collected, and the solvent and volatile matter were removed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com